ZOMEC via the p-Akt/Nrf2 Pathway Restored PTZ-Induced Oxidative Stress-Mediated Memory Dysfunction in Mouse Model
- PMID: 36193329
- PMCID: PMC9526611
- DOI: 10.1155/2022/8902262
ZOMEC via the p-Akt/Nrf2 Pathway Restored PTZ-Induced Oxidative Stress-Mediated Memory Dysfunction in Mouse Model
Retraction in
-
Retracted: ZOMEC via the p-Akt/Nrf2 Pathway Restored PTZ-Induced Oxidative Stress-Mediated Memory Dysfunction in Mouse Model.Biomed Res Int. 2024 Mar 20;2024:9893738. doi: 10.1155/2024/9893738. eCollection 2024. Biomed Res Int. 2024. PMID: 38549988 Free PMC article.
Abstract
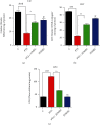
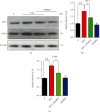
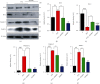
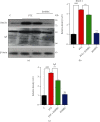
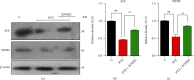
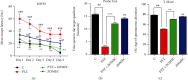
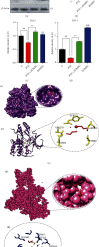
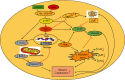
A new mechanistic approach to overcome the neurodegenerative disorders caused by oxidative stress in Alzheimer's disease (AD) is highly stressed in this article. Thus, a newly formulated drug (zinc ortho-methyl carbonodithioate (ZOMEC)) was investigated for five weeks on seven-week-old BALB/c male mice. ZOMEC 30 mg/kg was postadministered intraperitoneally during the third week of pentylenetetrazole (PTZ) injection. The brain homogenates of the mice were evaluated for their antioxidant potential for ZOMEC. The results including catalase (CAT), glutathione S transferase (GST), and lipid peroxidation (LPO) demonstrated that ZOMEC significantly reverted the oxidative stress stimulated by PTZ in the mouse brain. ZOMEC upregulated p-Akt/Nrf-2 pathways (also supported by molecular docking methods) to revoke PTZ-induced apoptotic protein markers. ZOMEC reversed PTZ-induced neuronal synapse deficits, improved oxidative stress-aided memory impairment, and inhibited the amyloidogenic pathway in mouse brains. The results suggested the potential of ZOMEC as a new, safe, and neurotherapeutic agent to cure neurodegenerative disorders by decreasing AD-like neuropathology in the animal PTZ model.
Copyright © 2022 Rifat Jahan et al.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Zinc Ortho Methyl Carbonodithioate Improved Pre and Post-Synapse Memory Impairment via SIRT1/p-JNK Pathway against Scopolamine in Adult Mice.J Neuroimmune Pharmacol. 2023 Jun;18(1-2):183-194. doi: 10.1007/s11481-023-10067-w. Epub 2023 Jun 1. J Neuroimmune Pharmacol. 2023. PMID: 37261605
-
Protective effect of sargahydroquinoic acid against Aβ25-35-evoked damage via PI3K/Akt mediated Nrf2 antioxidant defense system.Biomed Pharmacother. 2021 Dec;144:112271. doi: 10.1016/j.biopha.2021.112271. Epub 2021 Oct 4. Biomed Pharmacother. 2021. PMID: 34619494
-
Carveol Attenuates Seizure Severity and Neuroinflammation in Pentylenetetrazole-Kindled Epileptic Rats by Regulating the Nrf2 Signaling Pathway.Oxid Med Cell Longev. 2021 Aug 11;2021:9966663. doi: 10.1155/2021/9966663. eCollection 2021. Oxid Med Cell Longev. 2021. PMID: 34422216 Free PMC article.
-
Proanthocyanidins alleviate pentylenetetrazole-induced epileptic seizures in mice via the antioxidant activity.Neurochem Res. 2022 Oct;47(10):3012-3023. doi: 10.1007/s11064-022-03647-4. Epub 2022 Jul 15. Neurochem Res. 2022. PMID: 35838827
-
Natural Dietary Supplementation of Anthocyanins via PI3K/Akt/Nrf2/HO-1 Pathways Mitigate Oxidative Stress, Neurodegeneration, and Memory Impairment in a Mouse Model of Alzheimer's Disease.Mol Neurobiol. 2018 Jul;55(7):6076-6093. doi: 10.1007/s12035-017-0798-6. Epub 2017 Nov 23. Mol Neurobiol. 2018. PMID: 29170981
Cited by
-
Ranuncoside's attenuation of scopolamine-induced memory impairment in mice via Nrf2 and NF-ĸB signaling.Saudi Pharm J. 2023 Sep;31(9):101702. doi: 10.1016/j.jsps.2023.101702. Epub 2023 Jul 13. Saudi Pharm J. 2023. PMID: 37533493 Free PMC article.
-
Retracted: ZOMEC via the p-Akt/Nrf2 Pathway Restored PTZ-Induced Oxidative Stress-Mediated Memory Dysfunction in Mouse Model.Biomed Res Int. 2024 Mar 20;2024:9893738. doi: 10.1155/2024/9893738. eCollection 2024. Biomed Res Int. 2024. PMID: 38549988 Free PMC article.
References
-
- Kozlowski H., Janicka-Klos A., Brasun J., Gaggelli E., Valensin D., Valensin G. Copper, iron, and zinc ions homeostasis and their role in neurodegenerative disorders (metal uptake, transport, distribution and regulation) Coordination Chemistry Reviews . 2009;253(21-22):2665–2685. doi: 10.1016/j.ccr.2009.05.011. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

