Enhanced fear limits behavioral flexibility in Shank2-deficient mice
- PMID: 36192805
- PMCID: PMC9531513
- DOI: 10.1186/s13229-022-00518-1
Enhanced fear limits behavioral flexibility in Shank2-deficient mice
Abstract
Background: A core symptom of autism spectrum disorder (ASD) is repetitive and restrictive patterns of behavior. Cognitive inflexibility has been proposed as a potential basis for these symptoms of ASD. More generally, behavioral inflexibility has been proposed to underlie repetitive and restrictive behavior in ASD. Here, we investigated whether and how behavioral flexibility is compromised in a widely used animal model of ASD.
Methods: We compared the behavioral performance of Shank2-knockout mice and wild-type littermates in reversal learning employing a probabilistic classical trace conditioning paradigm. A conditioned stimulus (odor) was paired with an unconditioned appetitive (water, 6 µl) or aversive (air puff) stimulus in a probabilistic manner. We also compared air puff-induced eye closure responses of Shank2-knockout and wild-type mice.
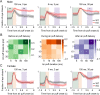
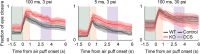
Results: Male, but not female, Shank2-knockout mice showed impaired reversal learning when the expected outcomes consisted of a water reward and a strong air puff. Moreover, male, but not female, Shank2-knockout mice showed stronger anticipatory eye closure responses to the air puff compared to wild-type littermates, raising the possibility that the impairment might reflect enhanced fear. In support of this contention, male Shank2-knockout mice showed intact reversal learning when the strong air puff was replaced with a mild air puff and when the expected outcomes consisted of only rewards.
Limitations: We examined behavioral flexibility in one behavioral task (reversal learning in a probabilistic classical trace conditioning paradigm) using one ASD mouse model (Shank2-knockout mice). Thus, future work is needed to clarify the extent to which our findings (that enhanced fear limits behavioral flexibility in ASD) can explain the behavioral inflexibility associated with ASD. Also, we examined only the relationship between fear and behavioral flexibility, leaving open the question of whether abnormalities in processes other than fear contribute to behavioral inflexibility in ASD. Finally, the neurobiological mechanisms linking Shank2-knockout and enhanced fear remain to be elucidated.
Conclusions: Our results indicate that enhanced fear suppresses reversal learning in the presence of an intact capability to learn cue-outcome contingency changes in Shank2-knockout mice. Our findings suggest that behavioral flexibility might be seriously limited by abnormal emotional responses in ASD.
Keywords: Classical conditioning; Fear; Reversal learning; Shank2.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures







Similar articles
-
Sex-specific modulation of safety learning in Shank2-deficient mice.Prog Neuropsychopharmacol Biol Psychiatry. 2024 Jun 8;132:110973. doi: 10.1016/j.pnpbp.2024.110973. Epub 2024 Feb 17. Prog Neuropsychopharmacol Biol Psychiatry. 2024. PMID: 38369099
-
Probing cognitive flexibility in Shank2-deficient mice: Effects of D-cycloserine and NMDAR signaling hub dynamics.Prog Neuropsychopharmacol Biol Psychiatry. 2024 Aug 30;134:111051. doi: 10.1016/j.pnpbp.2024.111051. Epub 2024 Jun 5. Prog Neuropsychopharmacol Biol Psychiatry. 2024. PMID: 38849086
-
Delayed reversal learning and association with repetitive behavior in autism spectrum disorders.Autism Res. 2012 Dec;5(6):398-406. doi: 10.1002/aur.1255. Epub 2012 Oct 24. Autism Res. 2012. PMID: 23097376
-
Behavioral phenotypes and neurobiological mechanisms in the Shank1 mouse model for autism spectrum disorder: A translational perspective.Behav Brain Res. 2018 Oct 15;352:46-61. doi: 10.1016/j.bbr.2017.09.038. Epub 2017 Sep 28. Behav Brain Res. 2018. PMID: 28963042 Review.
-
Cued and Contextual Fear Conditioning for Rodents.In: Buccafusco JJ, editor. Methods of Behavior Analysis in Neuroscience. 2nd edition. Boca Raton (FL): CRC Press/Taylor & Francis; 2009. Chapter 2. In: Buccafusco JJ, editor. Methods of Behavior Analysis in Neuroscience. 2nd edition. Boca Raton (FL): CRC Press/Taylor & Francis; 2009. Chapter 2. PMID: 21204331 Free Books & Documents. Review.
Cited by
-
Rodent Models for ASD Biomarker Development.Adv Neurobiol. 2024;40:189-218. doi: 10.1007/978-3-031-69491-2_8. Adv Neurobiol. 2024. PMID: 39562446 Review.
References
-
- American Psychiatric Association: Diagnostic and statistical manual of mental disorders (5th. ed). 2013.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

