Type 1 and Type 2 Epstein-Barr viruses induce proliferation, and inhibit differentiation, in infected telomerase-immortalized normal oral keratinocytes
- PMID: 36190982
- PMCID: PMC9529132
- DOI: 10.1371/journal.ppat.1010868
Type 1 and Type 2 Epstein-Barr viruses induce proliferation, and inhibit differentiation, in infected telomerase-immortalized normal oral keratinocytes
Abstract
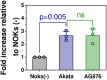
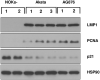
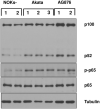
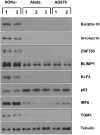
Differentiated epithelial cells are an important source of infectious EBV virions in human saliva, and latent Epstein-Barr virus (EBV) infection is strongly associated with the epithelial cell tumor, nasopharyngeal carcinoma (NPC). However, it has been difficult to model how EBV contributes to NPC, since EBV has not been shown to enhance proliferation of epithelial cells in monolayer culture in vitro and is not stably maintained in epithelial cells without antibiotic selection. In addition, although there are two major types of EBV (type 1 (T1) and type 2 (T2)), it is currently unknown whether T1 and T2 EBV behave differently in epithelial cells. Here we inserted a G418 resistance gene into the T2 EBV strain, AG876, allowing us to compare the phenotypes of T1 Akata virus versus T2 AG876 virus in a telomerase-immortalized normal oral keratinocyte cell line (NOKs) using a variety of different methods, including RNA-seq analysis, proliferation assays, immunoblot analyses, and air-liquid interface culture. We show that both T1 Akata virus infection and T2 AG876 virus infection of NOKs induce cellular proliferation, and inhibit spontaneous differentiation, in comparison to the uninfected cells when cells are grown without supplemental growth factors in monolayer culture. T1 EBV and T2 EBV also have a similar ability to induce epithelial-to-mesenchymal (EMT) transition and activate canonical and non-canonical NF-κB signaling in infected NOKs. In contrast to our recent results in EBV-infected lymphoblastoid cells (in which T2 EBV infection is much more lytic than T1 EBV infection), we find that NOKs infected with T1 and T2 EBV respond similarly to lytic inducing agents such as TPA treatment or differentiation. These results suggest that T1 and T2 EBV have similar phenotypes in infected epithelial cells, with both EBV types enhancing cellular proliferation and inhibiting differentiation when growth factors are limiting.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
Epstein-Barr virus LMP1 protein promotes proliferation and inhibits differentiation of epithelial cells via activation of YAP and TAZ.Proc Natl Acad Sci U S A. 2023 May 16;120(20):e2219755120. doi: 10.1073/pnas.2219755120. Epub 2023 May 8. Proc Natl Acad Sci U S A. 2023. PMID: 37155846 Free PMC article.
-
Epstein-Barr Virus Infection Promotes Epithelial Cell Growth by Attenuating Differentiation-Dependent Exit from the Cell Cycle.mBio. 2019 Aug 20;10(4):e01332-19. doi: 10.1128/mBio.01332-19. mBio. 2019. PMID: 31431547 Free PMC article.
-
B cells infected with Type 2 Epstein-Barr virus (EBV) have increased NFATc1/NFATc2 activity and enhanced lytic gene expression in comparison to Type 1 EBV infection.PLoS Pathog. 2020 Feb 14;16(2):e1008365. doi: 10.1371/journal.ppat.1008365. eCollection 2020 Feb. PLoS Pathog. 2020. PMID: 32059024 Free PMC article.
-
EBV Infection and Glucose Metabolism in Nasopharyngeal Carcinoma.Adv Exp Med Biol. 2017;1018:75-90. doi: 10.1007/978-981-10-5765-6_6. Adv Exp Med Biol. 2017. PMID: 29052133 Review.
-
Epstein-Barr virus infection and nasopharyngeal carcinoma.Philos Trans R Soc Lond B Biol Sci. 2017 Oct 19;372(1732):20160270. doi: 10.1098/rstb.2016.0270. Philos Trans R Soc Lond B Biol Sci. 2017. PMID: 28893937 Free PMC article. Review.
Cited by
-
Latent Epstein-Barr virus infection collaborates with Myc over-expression in normal human B cells to induce Burkitt-like Lymphomas in mice.PLoS Pathog. 2024 Apr 15;20(4):e1012132. doi: 10.1371/journal.ppat.1012132. eCollection 2024 Apr. PLoS Pathog. 2024. PMID: 38620028 Free PMC article.
-
The viral etiology of EBV-associated gastric cancers contributes to their unique pathology, clinical outcomes, treatment responses and immune landscape.Front Immunol. 2024 Mar 26;15:1358511. doi: 10.3389/fimmu.2024.1358511. eCollection 2024. Front Immunol. 2024. PMID: 38596668 Free PMC article. Review.
-
Epstein-Barr virus LMP1 protein promotes proliferation and inhibits differentiation of epithelial cells via activation of YAP and TAZ.Proc Natl Acad Sci U S A. 2023 May 16;120(20):e2219755120. doi: 10.1073/pnas.2219755120. Epub 2023 May 8. Proc Natl Acad Sci U S A. 2023. PMID: 37155846 Free PMC article.
-
Shared and distinct interactions of type 1 and type 2 Epstein-Barr Nuclear Antigen 2 with the human genome.BMC Genomics. 2024 Mar 12;25(1):273. doi: 10.1186/s12864-024-10183-8. BMC Genomics. 2024. PMID: 38475709 Free PMC article.
References
-
- Kieff E, Cohen JI, Longnecker R. Epstein-Barr Virus/Replication and Epstein-Barr Virus. 6th ed. In: Knipe DM, Howley PM, editors. Fields Virology. 6th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2013. pp. 1898–1959.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

