A brief insight into the etiology, genetics, and immunology of polycystic ovarian syndrome (PCOS)
- PMID: 36190593
- PMCID: PMC9723082
- DOI: 10.1007/s10815-022-02625-7
A brief insight into the etiology, genetics, and immunology of polycystic ovarian syndrome (PCOS)
Abstract
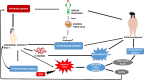
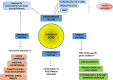
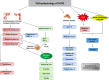
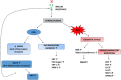
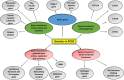
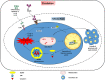
Polycystic ovarian syndrome (PCOS) is a prevailing endocrine and metabolic disorder occurring in about 6-20% of females in reproductive age. Most symptoms of PCOS arise early during puberty. Since PCOS involves a combination of signs and symptoms, thus it is considered as a heterogeneous disorderliness. The most accepted diagnostic criteria is Rotterdam criteria which involves two of the latter three features: (a) hyperandrogenism, (b) oligo- or an-ovulation, and (c) polycystic ovaries. The persistent hormonal imbalance leads to multiple small antral follicles formation and irregular menstrual cycle, ultimately causing infertility among females. Insulin resistance, cardiovascular diseases, abdominal obesity, psychological disorders, infertility, and cancer are also related to PCOS. These pathophysiologies associated with PCOS are interrelated with each other. Hyperandrogenism causes insulin resistance and hyperglycemia, leading to ROS formation, oxidative stress, and abdominal adiposity. In consequence, inflammation, ROS production, insulin resistance, and hyperandrogenemia also increase. Elevation of AGEs in the body either produced endogenously or consumed from diet exaggerates PCOS symptoms and is also related to ovarian dysfunction. This review summarizes how AGE formation, inflammation, and oxidative stress are significantly essential in PCOS progression. Alterations during prenatal development like exposure to excess AMH, androgens, or toxins (bisphenol-A, endocrine disruptors, etc.) may also be the etiologic mechanism behind PCOS. Although the etiology of this disorder is unclear, environmental and genetic factors are primarily involved. Physical inactivity, as well as unhealthy eating habits, has a vital role in the progression of PCOS. This review outlines a collection of specific genes phenotypically linked with PCOS. Furthermore, beneficial effect of metformin in maintaining endocrine abnormalities and ovarian function is also mentioned. Kisspeptin is a protein which helps in onset of puberty and increases GnRH pulsatile release during ovulation as well as role of KNDy neurons in GnRH pulsatile signal required for reproduction are also elaborated. This review also focuses on the immunology related to PCOS involving chronic low-grade inflammation, and how the alterations within the follicular microenvironment are intricated in the development of infertility in PCOS patients. How PCOS develops following antiepileptic and psychiatric medication is also expanded in this review. Initiation of antiandrogen treatment in early age (≤ 25 years) might be helpful in spontaneous conception in PCOS women. The role of BMP (bone morphogenetic proteins) in folliculogenesis and their expression in oocytes and granulosa cells are also explained. GDF8 and SERPINE1 expression in PCOS is given in detail.
Keywords: AMH; Advanced glycation end products (AGEs); Antiandrogen treatment; BMP (bone morphogenetic proteins); Chronic low-grade inflammation; GDF8; Genetics; Hyperandrogenism; Insulin resistance; Kisspeptin; Metformin; Oxidative stress; Polycystic ovarian syndrome; SERPINE1.
© 2022. The Author(s), under exclusive licence to Springer Science+Business Media, LLC, part of Springer Nature.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
AMERICAN ASSOCIATION OF CLINICAL ENDOCRINOLOGISTS, AMERICAN COLLEGE OF ENDOCRINOLOGY, AND ANDROGEN EXCESS AND PCOS SOCIETY DISEASE STATE CLINICAL REVIEW: GUIDE TO THE BEST PRACTICES IN THE EVALUATION AND TREATMENT OF POLYCYSTIC OVARY SYNDROME--PART 1.Endocr Pract. 2015 Nov;21(11):1291-300. doi: 10.4158/EP15748.DSC. Endocr Pract. 2015. PMID: 26509855
-
AMERICAN ASSOCIATION OF CLINICAL ENDOCRINOLOGISTS, AMERICAN COLLEGE OF ENDOCRINOLOGY, AND ANDROGEN EXCESS AND PCOS SOCIETY DISEASE STATE CLINICAL REVIEW: GUIDE TO THE BEST PRACTICES IN THE EVALUATION AND TREATMENT OF POLYCYSTIC OVARY SYNDROME - PART 2.Endocr Pract. 2015 Dec;21(12):1415-26. doi: 10.4158/EP15748.DSCPT2. Endocr Pract. 2015. PMID: 26642102 Review.
-
Characterization of reproductive, metabolic, and endocrine features of polycystic ovary syndrome in female hyperandrogenic mouse models.Endocrinology. 2014 Aug;155(8):3146-59. doi: 10.1210/en.2014-1196. Epub 2014 May 30. Endocrinology. 2014. PMID: 24877633
-
Source and amount of carbohydrate in the diet and inflammation in women with polycystic ovary syndrome.Nutr Res Rev. 2018 Dec;31(2):291-301. doi: 10.1017/S0954422418000136. Epub 2018 Jul 23. Nutr Res Rev. 2018. PMID: 30033891 Review.
-
At the crossroads of fertility and metabolism: the importance of AMPK-dependent signaling in female infertility associated with hyperandrogenism.Hum Reprod. 2022 May 30;37(6):1207-1228. doi: 10.1093/humrep/deac067. Hum Reprod. 2022. PMID: 35459945
Cited by
-
Multiple Benefits of Empagliflozin in PCOS: Evidence from a Preclinical Rat Model.Pathophysiology. 2024 Oct 9;31(4):559-582. doi: 10.3390/pathophysiology31040041. Pathophysiology. 2024. PMID: 39449523 Free PMC article.
-
Polycystic ovarian syndrome (PCOS) and recurrent spontaneous abortion (RSA) are associated with the PI3K-AKT pathway activation.PeerJ. 2024 Sep 6;12:e17950. doi: 10.7717/peerj.17950. eCollection 2024. PeerJ. 2024. PMID: 39253602 Free PMC article.
-
Androgen excess: a hallmark of polycystic ovary syndrome.Front Endocrinol (Lausanne). 2023 Dec 13;14:1273542. doi: 10.3389/fendo.2023.1273542. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 38152131 Free PMC article. Review.
-
Which is the current knowledge on man-made endocrine- disrupting chemicals in follicular fluid? An overview of effects on ovarian function and reproductive health.Front Endocrinol (Lausanne). 2024 Oct 2;15:1435121. doi: 10.3389/fendo.2024.1435121. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39415794 Free PMC article. Review.
-
Insights into Vitamin E with Combined Oral Contraceptive on INSR Gene in PCOS by Integrating In Silico and In Vivo Approaches.Appl Biochem Biotechnol. 2024 Jun;196(6):2990-3009. doi: 10.1007/s12010-023-04710-8. Epub 2023 Aug 23. Appl Biochem Biotechnol. 2024. PMID: 37610513
References
-
- A. Szilagyi and I. Szabo, “Endocrine characteristics of polycystic ovary syndrome (PCOS),” IJEB Vol.41(07) [July 2003], 2003, Accessed: Jan. 18, 2022. [Online]. Available: http://nopr.niscair.res.in/handle/123456789/17119 - PubMed
-
- R. Azziz et al., “Polycystic ovary syndrome,” Nature Reviews Disease Primers 2016 2:110.1038/nrdp.2016.57. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

