Utilizing mast cells in a positive manner to overcome inflammatory and allergic diseases
- PMID: 36189267
- PMCID: PMC9518231
- DOI: 10.3389/fimmu.2022.937120
Utilizing mast cells in a positive manner to overcome inflammatory and allergic diseases
Abstract
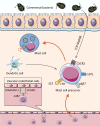
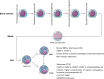
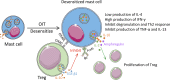
Mast cells (MCs) are immune cells widely distributed in the body, accompanied by diverse phenotypes and functions. Committed mast cell precursors (MCPs) leave the bone marrow and enter the blood circulation, homing to peripheral sites under the control of various molecules from different microenvironments, where they eventually differentiate and mature. Partly attributable to the unique maturation mechanism, MCs display high functional heterogeneity and potentially plastic phenotypes. High plasticity also means that MCs can exhibit different subtypes to cope with different microenvironments, which we call "the peripheral immune education system". Under the peripheral immune education system, MCs showed a new character from previous cognition in some cases, namely regulation of allergy and inflammation. In this review, we focus on the mucosal tissues, such as the gastrointestinal tract, to gain insights into the mechanism underlying the migration of MCs to the gut or other organs and their heterogeneity, which is driven by different microenvironments. In particular, the immunosuppressive properties of MCs let us consider that positively utilizing MCs may be a new way to overcome inflammatory and allergic disorders.
Keywords: allergy; heterogeneity; inflammation; mast cells; tolerance.
Copyright © 2022 Zhang, Ernst, Kiyono and Kurashima.
Conflict of interest statement
HK is director and founder of HanaVax Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Is it time for a new classification of mast cells? What do we know about mast cell heterogeneity?Immunol Rev. 2018 Mar;282(1):35-46. doi: 10.1111/imr.12636. Immunol Rev. 2018. PMID: 29431204 Review.
-
Neuro-allergology: Mast cell-nerve cross-talk.Allergol Int. 2022 Jul;71(3):288-293. doi: 10.1016/j.alit.2022.04.002. Epub 2022 Jun 7. Allergol Int. 2022. PMID: 35688775 Review.
-
Mast cells: ontogeny, homing, and recruitment of a unique innate effector cell.J Allergy Clin Immunol. 2006 Jun;117(6):1285-91. doi: 10.1016/j.jaci.2006.04.017. J Allergy Clin Immunol. 2006. PMID: 16750988 Review.
-
Infiltration and local differentiation of bone marrow-derived integrinβ7-positive mast cell progenitors in atopic dermatitis-like skin.J Allergy Clin Immunol. 2023 Jan;151(1):159-171.e8. doi: 10.1016/j.jaci.2022.09.011. Epub 2022 Sep 17. J Allergy Clin Immunol. 2023. PMID: 36122789
-
Dermal Extracellular Matrix-Derived Hydrogels as an In Vitro Substrate to Study Mast Cell Maturation.Tissue Eng Part A. 2021 Aug;27(15-16):1008-1022. doi: 10.1089/ten.TEA.2020.0142. Epub 2020 Nov 19. Tissue Eng Part A. 2021. PMID: 33003982 Free PMC article.
Cited by
-
Exploring Mast Cell-CD8 T Cell Interactions in Inflammatory Skin Diseases.Int J Mol Sci. 2023 Jan 13;24(2):1564. doi: 10.3390/ijms24021564. Int J Mol Sci. 2023. PMID: 36675078 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

