Endogenous advanced glycation end products in the pathogenesis of chronic diabetic complications
- PMID: 36188225
- PMCID: PMC9521189
- DOI: 10.3389/fmolb.2022.1002710
Endogenous advanced glycation end products in the pathogenesis of chronic diabetic complications
Abstract
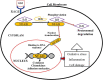
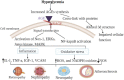
Diabetes is a common metabolic illness characterized by hyperglycemia and is linked to long-term vascular problems that can impair the kidney, eyes, nerves, and blood vessels. By increasing protein glycation and gradually accumulating advanced glycation end products in the tissues, hyperglycemia plays a significant role in the pathogenesis of diabetic complications. Advanced glycation end products are heterogeneous molecules generated from non-enzymatic interactions of sugars with proteins, lipids, or nucleic acids via the glycation process. Protein glycation and the buildup of advanced glycation end products are important in the etiology of diabetes sequelae such as retinopathy, nephropathy, neuropathy, and atherosclerosis. Their contribution to diabetes complications occurs via a receptor-mediated signaling cascade or direct extracellular matrix destruction. According to recent research, the interaction of advanced glycation end products with their transmembrane receptor results in intracellular signaling, gene expression, the release of pro-inflammatory molecules, and the production of free radicals, all of which contribute to the pathology of diabetes complications. The primary aim of this paper was to discuss the chemical reactions and formation of advanced glycation end products, the interaction of advanced glycation end products with their receptor and downstream signaling cascade, and molecular mechanisms triggered by advanced glycation end products in the pathogenesis of both micro and macrovascular complications of diabetes mellitus.
Keywords: advanced glycation end products; diabetes complication; glycation; hyperglycemia; receptor advanced glycation end products.
Copyright © 2022 Mengstie, Chekol Abebe, Behaile Teklemariam, Tilahun Mulu, Agidew, Teshome Azezew, Zewde and Agegnehu Teshome.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
AGEs accumulation with vascular complications, glycemic control and metabolic syndrome: A narrative review.Bone. 2023 Nov;176:116884. doi: 10.1016/j.bone.2023.116884. Epub 2023 Aug 18. Bone. 2023. PMID: 37598920 Review.
-
Advanced glycation end products and diabetic complications.Korean J Physiol Pharmacol. 2014 Feb;18(1):1-14. doi: 10.4196/kjpp.2014.18.1.1. Epub 2014 Feb 13. Korean J Physiol Pharmacol. 2014. PMID: 24634591 Free PMC article. Review.
-
Advanced glycation endproducts--role in pathology of diabetic complications.Diabetes Res Clin Pract. 2005 Jan;67(1):3-21. doi: 10.1016/j.diabres.2004.09.004. Diabetes Res Clin Pract. 2005. PMID: 15620429 Review.
-
Advanced Glycation End Products and Diabetes Mellitus: Mechanisms and Perspectives.Biomolecules. 2022 Apr 4;12(4):542. doi: 10.3390/biom12040542. Biomolecules. 2022. PMID: 35454131 Free PMC article. Review.
-
The Mechanisms of Inhibition of Advanced Glycation End Products Formation through Polyphenols in Hyperglycemic Condition.Planta Med. 2016 Jan;82(1-2):32-45. doi: 10.1055/s-0035-1558086. Epub 2015 Nov 9. Planta Med. 2016. PMID: 26550791 Review.
Cited by
-
Discovery of dual-action phenolic 4-arylidene-isoquinolinones with antioxidant and α-glucosidase inhibition activities.RSC Med Chem. 2023 Dec 4;15(2):519-538. doi: 10.1039/d3md00585b. eCollection 2024 Feb 21. RSC Med Chem. 2023. PMID: 38389895 Free PMC article.
-
Neurohistopathological Alterations Induced by Theobroma Cacao and Camellia Sinensis Extracts in Diabetic Male Wistar Rats.Cureus. 2023 Nov 8;15(11):e48492. doi: 10.7759/cureus.48492. eCollection 2023 Nov. Cureus. 2023. PMID: 38073976 Free PMC article.
-
Facile fabrication of redox nanoparticles loaded with exosomal-miRNAs and resveratrol as glycation inhibitor in alleviating the progression and development of diabetic cataract.Naunyn Schmiedebergs Arch Pharmacol. 2024 Oct 24. doi: 10.1007/s00210-024-03535-4. Online ahead of print. Naunyn Schmiedebergs Arch Pharmacol. 2024. PMID: 39446149
-
Flavonols as a Potential Pharmacological Intervention for Alleviating Cognitive Decline in Diabetes: Evidence from Preclinical Studies.Life (Basel). 2023 Nov 30;13(12):2291. doi: 10.3390/life13122291. Life (Basel). 2023. PMID: 38137892 Free PMC article. Review.
-
Association of Zinc Status with Matrix Metalloproteinases, Advanced Glycation End-Products, and Blood Pressure in Patients with Chronic Kidney Disease.Biol Trace Elem Res. 2023 Sep;201(9):4275-4285. doi: 10.1007/s12011-022-03524-9. Epub 2022 Dec 14. Biol Trace Elem Res. 2023. PMID: 36515817
References
Publication types
LinkOut - more resources
Full Text Sources

