Increased mast cell activation in eosinophilic chronic obstructive pulmonary disease
- PMID: 36188122
- PMCID: PMC9512688
- DOI: 10.1002/cti2.1417
Increased mast cell activation in eosinophilic chronic obstructive pulmonary disease
Abstract
Objectives: A subset of chronic obstructive pulmonary disease (COPD) patients have increased numbers of airway eosinophils associated with elevated markers of T2 inflammation. This analysis focussed on mast cell counts and mast cell-related gene expression in COPD patients with higher vs lower eosinophil counts.
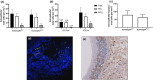
Methods: We investigated gene expression of tryptase (TPSAB1), carboxypeptidase A3 (CPA3), chymase (CMA1) and two mast cell specific gene signatures; a bronchial biopsy signature (MCbb) and an IgE signature (MCIgE) using sputum cells and bronchial epithelial brushings. Gene expression analysis was conducted by RNA-sequencing. We also examined bronchial biopsy mast cell numbers by immunohistochemistry.
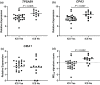
Results: There was increased expression of TPSAB1, CPA3 and MCbb in eosinophilhigh than in eosinophillow COPD patients in sputum cells and bronchial epithelial brushings (fold change differences 1.21 and 1.28, respectively, P < 0.01). Mast cell gene expression was associated with markers of T2 and eosinophilic inflammation (IL13, CLCA1, CST1, CCL26, eosinophil counts in sputum and bronchial mucosa; rho = 0.4-0.8; P < 0.05). There was no difference in MCIgE gene expression between groups. There was no difference in the total number of bronchial biopsy mast cells between groups.
Conclusion: These results demonstrate that eosinophilic inflammation is associated with altered mast cell characteristics in COPD patients, implicating mast cells as a component of T2 inflammation present in a subset of COPD patients.
Keywords: eosinophils; epithelial cells; sputum; type 2 inflammation.
© 2022 The Authors. Clinical & Translational Immunology published by John Wiley & Sons Australia, Ltd on behalf of Australian and New Zealand Society for Immunology, Inc.
Conflict of interest statement
DS has received sponsorship to attend and speak at international meetings, honoraria for lecturing or attending advisory boards from the following companies: Aerogen, AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, CSL Behring, Epiendo, Genentech, GlaxoSmithKline, Glenmark, Gossamerbio, Kinaset, Menarini, Novartis, Pulmatrix, Sanofi, Teva, Theravance and Verona. AH and JD have no conflicts of interest. T‐HP and CM are employees of AstraZeneca.
Figures



Similar articles
-
Sputum mast cell subtypes relate to eosinophilia and corticosteroid response in asthma.Eur Respir J. 2016 Apr;47(4):1123-33. doi: 10.1183/13993003.01098-2015. Epub 2015 Dec 23. Eur Respir J. 2016. PMID: 26699720
-
Sputum Gene Expression Reveals Dysregulation of Mast Cells and Basophils in Eosinophilic COPD.Int J Chron Obstruct Pulmon Dis. 2021 Jul 21;16:2165-2179. doi: 10.2147/COPD.S305380. eCollection 2021. Int J Chron Obstruct Pulmon Dis. 2021. PMID: 34321876 Free PMC article.
-
Molecular markers of type 2 airway inflammation are similar between eosinophilic severe asthma and eosinophilic chronic obstructive pulmonary disease.Allergy. 2021 Jul;76(7):2079-2089. doi: 10.1111/all.14741. Epub 2021 Mar 4. Allergy. 2021. PMID: 33470427
-
Eosinophils in COPD-Current Concepts and Clinical Implications.J Allergy Clin Immunol Pract. 2020 Sep;8(8):2565-2574. doi: 10.1016/j.jaip.2020.03.017. Epub 2020 Apr 3. J Allergy Clin Immunol Pract. 2020. PMID: 32251737 Review.
-
Role of IgE in the development of allergic airway inflammation and airway hyperresponsiveness--a murine model.Allergy. 1999 Apr;54(4):297-305. doi: 10.1034/j.1398-9995.1999.00085.x. Allergy. 1999. PMID: 10371087 Review.
Cited by
-
How inhaled corticosteroids target inflammation in COPD.Eur Respir Rev. 2023 Oct 18;32(170):230084. doi: 10.1183/16000617.0084-2023. Print 2023 Dec 31. Eur Respir Rev. 2023. PMID: 37852657 Free PMC article. Review.
-
Type 2 inflammation in COPD: is it just asthma?Breathe (Sheff). 2024 Nov 12;20(3):230229. doi: 10.1183/20734735.0229-2023. eCollection 2024 Oct. Breathe (Sheff). 2024. PMID: 39534492 Free PMC article. Review.
-
Current smoking reduces small airway eosinophil counts in COPD.ERJ Open Res. 2024 Jan 22;10(1):00870-2023. doi: 10.1183/23120541.00870-2023. eCollection 2024 Jan. ERJ Open Res. 2024. PMID: 38259811 Free PMC article.
-
HucMSC-Ex alleviates DSS-induced colitis in mice by decreasing mast cell activation via the IL-33/ST2 axis.Am J Transl Res. 2024 Jun 25;16(6):2727-2744. doi: 10.62347/EXZE5413. eCollection 2024. Am J Transl Res. 2024. PMID: 39006299 Free PMC article.
-
Type 2 Cystatins and Their Roles in the Regulation of Human Immune Response and Cancer Progression.Cancers (Basel). 2023 Nov 10;15(22):5363. doi: 10.3390/cancers15225363. Cancers (Basel). 2023. PMID: 38001623 Free PMC article. Review.
References
-
- Rutgers SR, Timens W, Kaufmann HF, van der Mark TW, Koeter GH, Postma DS. Comparison of induced sputum with bronchial wash, bronchoalveolar lavage and bronchial biopsies in COPD. Eur Respir J 2000; 15: 109–115. - PubMed
-
- Higham A, Leow‐Dyke S, Jackson N, Singh D. Stability of eosinophilic inflammation in COPD bronchial biopsies. Eur Respir J 2020; 56: 2004167. - PubMed
-
- Kolsum U, Damera G, Pham TH et al. Pulmonary inflammation in patients with chronic obstructive pulmonary disease with higher blood eosinophil counts. J Allergy Clin Immunol 2017; 140: 1181–1184.e7. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
