Current Review in Basic Science: Animal Models of Focal Cortical Dysplasia and Epilepsy
- PMID: 36187145
- PMCID: PMC9483763
- DOI: 10.1177/15357597221098230
Current Review in Basic Science: Animal Models of Focal Cortical Dysplasia and Epilepsy
Abstract
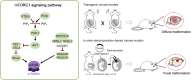
Focal cortical dysplasia (FCD) is a malformation of cortical development that is a prevalent cause of intractable epilepsy in children. Of the three FCD subtypes, understanding the etiology and pathogenesis of FCD type II has seen the most progress owing to the recent advances in identifying gene mutations along the mTOR signaling pathway as a frequent cause of this disorder. Accordingly, numerous animal models of FCD type II based on genetic manipulation of the mTOR signaling pathway have emerged to investigate the mechanisms of epileptogenesis and novel therapeutics for epilepsy. These include transgenic and in utero electroporation-based animal models. Here, we review the histopathological and electroclinical features of existing FCD type II animal models and discuss the scientific and technical considerations, clinical applications, and limitations of current models. We also highlight other models of FCD based on early life acquired factors.
Keywords: CRISPR/Cas9; astrocytes; cortical development; cortical lesion; in utero electroporation; malformation of cortical development; pyramidal neurons; seizure; transgenic mice.
© The Author(s) 2022.
Conflict of interest statement
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures
Similar articles
-
Focal cortical dysplasia pathology: diagnostic difficulty, classification, and utility for pathogenesis.Neurosurg Focus. 2022 Oct;53(4):E6. doi: 10.3171/2022.7.FOCUS21731. Neurosurg Focus. 2022. PMID: 36183176
-
Brain somatic mutations in MTOR leading to focal cortical dysplasia.BMB Rep. 2016 Feb;49(2):71-2. doi: 10.5483/bmbrep.2016.49.2.010. BMB Rep. 2016. PMID: 26779999 Free PMC article.
-
Clinical, imaging, and immunohistochemical characteristics of focal cortical dysplasia Type II extratemporal epilepsies in children: analyses of an institutional case series.J Neurosurg Pediatr. 2017 Feb;19(2):182-195. doi: 10.3171/2016.8.PEDS1686. Epub 2016 Nov 25. J Neurosurg Pediatr. 2017. PMID: 27885945
-
Focal cortical dysplasia: etiology, epileptogenesis, classification, clinical presentation, imaging, and management.Childs Nerv Syst. 2020 Dec;36(12):2939-2947. doi: 10.1007/s00381-020-04851-9. Epub 2020 Aug 6. Childs Nerv Syst. 2020. PMID: 32766946 Review.
-
SLC35A2 somatic variants in drug resistant epilepsy: FCD and MOGHE.Neurobiol Dis. 2023 Oct 15;187:106299. doi: 10.1016/j.nbd.2023.106299. Epub 2023 Sep 20. Neurobiol Dis. 2023. PMID: 37739137 Free PMC article. Review.
Cited by
-
The mTOR pathway genes mTOR, Rheb, Depdc5, Pten, and Tsc1 have convergent and divergent impacts on cortical neuron development and function.bioRxiv [Preprint]. 2024 Jan 6:2023.08.11.553034. doi: 10.1101/2023.08.11.553034. bioRxiv. 2024. Update in: Elife. 2024 Feb 27;12:RP91010. doi: 10.7554/eLife.91010. PMID: 37609221 Free PMC article. Updated. Preprint.
-
Multi-tensor diffusion abnormalities of gray matter in an animal model of cortical dysplasia.Front Neurol. 2023 May 5;14:1124282. doi: 10.3389/fneur.2023.1124282. eCollection 2023. Front Neurol. 2023. PMID: 37342776 Free PMC article.
-
mTOR and neuroinflammation in epilepsy: implications for disease progression and treatment.Nat Rev Neurosci. 2024 May;25(5):334-350. doi: 10.1038/s41583-024-00805-1. Epub 2024 Mar 26. Nat Rev Neurosci. 2024. PMID: 38531962 Review.
-
The mTOR pathway genes MTOR, Rheb, Depdc5, Pten, and Tsc1 have convergent and divergent impacts on cortical neuron development and function.Elife. 2024 Feb 27;12:RP91010. doi: 10.7554/eLife.91010. Elife. 2024. PMID: 38411613 Free PMC article.
References
-
- Harvey AS, Cross JH, Shinnar S, Mathern GW, Taskforce IPESS. Defining the spectrum of international practice in pediatric epilepsy surgery patients. Epilepsia. 2008;49:146-155. doi:10.1111/j.1528-1167.2007.01421.x. - PubMed
-
- Blumcke I, Spreafico R, Haaker G, et al. Histopathological findings in brain tissue obtained during epilepsy surgery. N Engl J Med. 2017;377:1648-1656. doi:10.1056/NEJMoa1703784. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

