Selective inhibition of soluble tumor necrosis factor signaling reduces abdominal aortic aneurysm progression
- PMID: 36186984
- PMCID: PMC9523116
- DOI: 10.3389/fcvm.2022.942342
Selective inhibition of soluble tumor necrosis factor signaling reduces abdominal aortic aneurysm progression
Erratum in
-
Corrigendum: Selective inhibition of soluble tumor necrosis factor signaling reduces abdominal aortic aneurysm progression.Front Cardiovasc Med. 2024 Aug 19;11:1466614. doi: 10.3389/fcvm.2024.1466614. eCollection 2024. Front Cardiovasc Med. 2024. PMID: 39224753 Free PMC article.
Abstract
Background: Tumor necrosis factor (TNF) is pathologically elevated in human abdominal aortic aneurysms (AAA). Non-selective TNF inhibition-based therapeutics are approved for human use but have been linked to several side effects. Compounds that target the proinflammatory soluble form of TNF (solTNF) but preserve the immunomodulatory capabilities of the transmembrane form of TNF (tmTNF) may prevent these side effects. We hypothesize that inhibition of solTNF signaling prevents AAA expansion.
Methods: The effect of the selective solTNF inhibitor, XPro1595, and the non-selective TNF inhibitor, Etanercept (ETN) was examined in porcine pancreatic elastase (PPE) induced AAA mice, and findings with XPro1595 was confirmed in angiotensin II (ANGII) induced AAA in hyperlipidemic apolipoprotein E (Apoe) -/- mice.
Results: XPro1595 treatment significantly reduced AAA expansion in both models, and a similar trend (p = 0.06) was observed in PPE-induced AAA in ETN-treated mice. In the PPE aneurysm wall, XPro1595 improved elastin integrity scores. In aneurysms, mean TNFR1 levels reduced non-significantly (p = 0.07) by 50% after TNF inhibition, but the histological location in murine AAAs was unaffected and similar to that in human AAAs. Semi-quantification of infiltrating leucocytes, macrophages, T-cells, and neutrophils in the aneurysm wall were unaffected by TNF inhibition. XPro1595 increased systemic TNF levels, while ETN increased systemic IL-10 levels. In ANGII-induced AAA mice, XPro1595 increased systemic TNF and IL-5 levels. In early AAA development, proteomic analyses revealed that XPro1595 significantly upregulated ontology terms including "platelet aggregation" and "coagulation" related to the fibrinogen complex, from which several proteins were among the top regulated proteins. Downregulated ontology terms were associated with metabolic processes.
Conclusion: In conclusion, selective inhibition of solTNF signaling reduced aneurysm expansion in mice, supporting its potential as an attractive treatment option for AAA patients.
Keywords: abdominal aortic aneurysm; cardiovascular disease; inflammation; translational research; tumor necrosis factor inhibitor; vascular inflammation.
Copyright © 2022 Griepke, Grupe, Lindholt, Fuglsang, Steffensen, Beck, Larsen, Bang-Møller, Overgaard, Rasmussen, Lambertsen and Stubbe.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
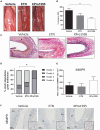
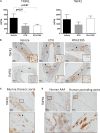

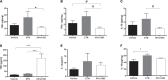

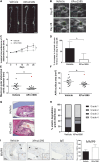
 ) and excluded from the statistical analyses. M, media; Ad, adventitia.
) and excluded from the statistical analyses. M, media; Ad, adventitia.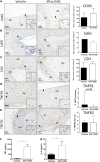
Similar articles
-
Hyperglycemia limits experimental aortic aneurysm progression.J Vasc Surg. 2010 Oct;52(4):975-83. doi: 10.1016/j.jvs.2010.05.086. Epub 2010 Aug 3. J Vasc Surg. 2010. PMID: 20678880 Free PMC article.
-
Castration of male mice prevents the progression of established angiotensin II-induced abdominal aortic aneurysms.J Vasc Surg. 2015 Mar;61(3):767-76. doi: 10.1016/j.jvs.2013.11.004. Epub 2014 Jan 16. J Vasc Surg. 2015. PMID: 24439319 Free PMC article.
-
PANoptosis in vascular smooth muscle cells regulated by TNF-α/IL-1β can be a new target for alleviating the progression of abdominal aortic aneurysm.Physiol Genomics. 2024 Feb 1;56(2):158-166. doi: 10.1152/physiolgenomics.00053.2023. Epub 2023 Dec 4. Physiol Genomics. 2024. PMID: 38047310
-
Inhibition or deletion of angiotensin II type 1 receptor suppresses elastase-induced experimental abdominal aortic aneurysms.J Vasc Surg. 2018 Feb;67(2):573-584.e2. doi: 10.1016/j.jvs.2016.12.110. Epub 2017 Apr 20. J Vasc Surg. 2018. PMID: 28434702 Free PMC article.
-
Central but not systemic administration of XPro1595 is therapeutic following moderate spinal cord injury in mice.J Neuroinflammation. 2014 Sep 10;11:159. doi: 10.1186/s12974-014-0159-6. J Neuroinflammation. 2014. PMID: 25204558 Free PMC article.
Cited by
-
TNF and TNF receptors as therapeutic targets for rheumatic diseases and beyond.Nat Rev Rheumatol. 2023 Sep;19(9):576-591. doi: 10.1038/s41584-023-01002-7. Epub 2023 Aug 4. Nat Rev Rheumatol. 2023. PMID: 37542139 Review.
-
Fn14 and TNFR2 as regulators of cytotoxic TNFR1 signaling.Front Cell Dev Biol. 2023 Nov 6;11:1267837. doi: 10.3389/fcell.2023.1267837. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 38020877 Free PMC article. Review.
-
Application and challenges of TCR and BCR sequencing to investigate T- and B-cell clonality in elastase-induced experimental murine abdominal aortic aneurysm.Front Cardiovasc Med. 2023 Nov 14;10:1221620. doi: 10.3389/fcvm.2023.1221620. eCollection 2023. Front Cardiovasc Med. 2023. PMID: 38034381 Free PMC article.
-
Pentagalloyl glucose induces anti-inflammatory macrophage polarization - suppressing macrophage mediated vascular cell dysfunction and TGF-β secretion.Int J Immunopathol Pharmacol. 2024 Jan-Dec;38:3946320241276894. doi: 10.1177/03946320241276894. Int J Immunopathol Pharmacol. 2024. PMID: 39135409 Free PMC article.
References
-
- Brewster DC, Cronenwett JL, Hallett JW, Jr., Johnston KW, Krupski WC, Matsumura JS. Guidelines for the treatment of abdominal aortic aneurysms. Report of a subcommittee of the joint council of the American association for vascular surgery and society for vascular surgery. J Vasc Surg. (2003) 37:1106–17. 10.1067/mva.2003.363 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

