Dental Pulp Stem Cell-Derived Conditioned Medium Alleviates Subarachnoid Hemorrhage-Induced Microcirculation Impairment by Promoting M2 Microglia Polarization and Reducing Astrocyte Swelling
- PMID: 36181630
- PMCID: PMC10444696
- DOI: 10.1007/s12975-022-01083-8
Dental Pulp Stem Cell-Derived Conditioned Medium Alleviates Subarachnoid Hemorrhage-Induced Microcirculation Impairment by Promoting M2 Microglia Polarization and Reducing Astrocyte Swelling
Abstract
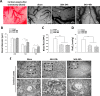
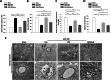
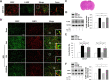
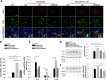
Aneurysmal subarachnoid hemorrhage (SAH) can cause severe neurological deficits and high mortality. Early brain edema following SAH contributes to the initiation of microcirculation impairment and may further lead to delayed ischemic neurologic deficit (DIND). This study aimed to investigate whether dental pulp stem cell conditioned medium (DPSC-CM) ameliorates SAH-induced microcirculation impairment and the underlying mechanisms. SAH was induced via intrathecal injection of fresh autologous blood in Wistar male adult rat. DPSC-CM or DPSC-CM + insulin growth factor-1 (IGF-1) antibody was randomly administered by intrathecal route 5 min after SAH induction. To evaluate the underlying mechanisms of DPSC-CM in the treatment of SAH, primary rat astrocyte and microglia co-cultures were challenged with hemolysate or SAH-patient CSF in the presence or absence of DPSC-CM. The results showed that in vivo, DPSC-CM treatment decreased the brain water content, improved microcirculation impairment and enhanced functional recovery at 24 h post-SAH. DPSC-CM treatment also alleviated the expressions of water channel protein aquaporin-4 (AQP4) and pro-inflammatory cytokines, and enhanced the expressions of anti-inflammatory factors in the cortical region. However, all the beneficial effects of DPSC-CM were abrogated after treatment with IGF-1 neutralizing antibody. The in vitro results further showed that DPSC-CM treatment reduced hemolysate/SAH-patient CSF-induced astrocyte swelling and promoted M2 microglia polarization, partially through IGF-1/AKT signaling. The data suggested that DPSC-CM significantly reduced brain edema and rescued microcirculation impairment with concomitant anti-inflammatory benefits after SAH, and may potentially be developed into a novel therapeutic strategy for SAH.
Keywords: Aneurysmal subarachnoid hemorrhage; Brain edema; Conditioned medium; Dental pulp stem cells; Microcirculation impairment; Neuroinflammation.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Dental Pulp Stem Cell-Derived Factors Alleviate Subarachnoid Hemorrhage-Induced Neuroinflammation and Ischemic Neurological Deficits.Int J Mol Sci. 2019 Jul 31;20(15):3747. doi: 10.3390/ijms20153747. Int J Mol Sci. 2019. PMID: 31370244 Free PMC article.
-
Recombinant soluble form of receptor for advanced glycation end products ameliorates microcirculation impairment and neuroinflammation after subarachnoid hemorrhage.Neurotherapeutics. 2024 Mar;21(2):e00312. doi: 10.1016/j.neurot.2023.e00312. Epub 2024 Jan 4. Neurotherapeutics. 2024. PMID: 38177024 Free PMC article.
-
Dental pulp mesenchymal stem cell-derived exosomes inhibit neuroinflammation and microglial pyroptosis in subarachnoid hemorrhage via the miRNA-197-3p/FOXO3 axis.J Nanobiotechnology. 2024 Jul 19;22(1):426. doi: 10.1186/s12951-024-02708-w. J Nanobiotechnology. 2024. PMID: 39030593 Free PMC article.
-
The role of the astrocyte in subarachnoid hemorrhage and its therapeutic implications.Front Immunol. 2022 Sep 29;13:1008795. doi: 10.3389/fimmu.2022.1008795. eCollection 2022. Front Immunol. 2022. PMID: 36248855 Free PMC article. Review.
-
Narrative review of roles of astrocytes in subarachnoid hemorrhage.Ann Transl Med. 2023 Apr 28;11(8):302. doi: 10.21037/atm-22-5486. Epub 2023 Mar 23. Ann Transl Med. 2023. PMID: 37181334 Free PMC article. Review.
Cited by
-
Dental pulp stem cells regenerate neural tissue in degenerative disorders and stroke rehabilitation: A scope systematic review.Heliyon. 2024 Jul 25;10(15):e35080. doi: 10.1016/j.heliyon.2024.e35080. eCollection 2024 Aug 15. Heliyon. 2024. PMID: 39166055 Free PMC article.
-
Remote Ischemic Post-conditioning Reduces Cognitive Impairment in Rats Following Subarachnoid Hemorrhage: Possible Involvement in STAT3/STAT5 Phosphorylation and Th17/Treg Cell Homeostasis.Transl Stroke Res. 2024 Feb 15. doi: 10.1007/s12975-024-01235-y. Online ahead of print. Transl Stroke Res. 2024. PMID: 38356020
-
Therapeutic potential of stem cells in subarachnoid hemorrhage.Neural Regen Res. 2025 Apr 1;20(4):936-945. doi: 10.4103/NRR.NRR-D-24-00124. Epub 2024 May 17. Neural Regen Res. 2025. PMID: 38989928 Free PMC article.
-
Functionalized Nanomaterials Capable of Crossing the Blood-Brain Barrier.ACS Nano. 2024 Jan 23;18(3):1820-1845. doi: 10.1021/acsnano.3c10674. Epub 2024 Jan 9. ACS Nano. 2024. PMID: 38193927 Free PMC article. Review.
-
Methods for studying mammalian aquaporin biology.Biol Methods Protoc. 2023 Nov 11;8(1):bpad031. doi: 10.1093/biomethods/bpad031. eCollection 2023. Biol Methods Protoc. 2023. PMID: 38046463 Free PMC article. Review.
References
-
- Macdonald RL, Schweizer TA. Spontaneous subarachnoid haemorrhage. Lancet. 2017;389(10069):655–666. - PubMed
-
- Rosengart AJ, Schultheiss KE, Tolentino J, Macdonald RL. Prognostic factors for outcome in patients with aneurysmal subarachnoid hemorrhage. Stroke. 2007;38(8):2315–2321. - PubMed
-
- Zetterling M, Hallberg L, Ronne-Engstrom E. Early global brain oedema in relation to clinical admission parameters and outcome in patients with aneurysmal subarachnoid haemorrhage. Acta Neurochir (Wien) 2010;152(9):1527–1533. - PubMed
-
- Sehba FA, Friedrich V. Cerebral microvasculature is an early target of subarachnoid hemorrhage. Acta Neurochir Suppl. 2013;115:199–205. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
