Dramatic impacts on brain pathology, anxiety, and cognitive function in the knock-in APPNL-G-F mouse model of Alzheimer disease following long-term voluntary exercise
- PMID: 36180883
- PMCID: PMC9526288
- DOI: 10.1186/s13195-022-01085-6
Dramatic impacts on brain pathology, anxiety, and cognitive function in the knock-in APPNL-G-F mouse model of Alzheimer disease following long-term voluntary exercise
Abstract
Background: An active lifestyle is associated with improved cognitive functions in aged people and may prevent or slow down the progression of various neurodegenerative diseases including Alzheimer's disease (AD). To investigate these protective effects, male APPNL-G-F mice were exposed to long-term voluntary exercise.
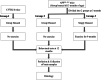
Methods: Three-month-old AD mice were housed in a cage supplemented with a running wheel for 9 months for long-term exercise. At the age of 12 months, behavioral tests were completed for all groups. After completing behavioral testing, their brains were assessed for amyloid pathology, microgliosis, and cholinergic cells.
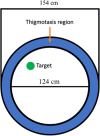
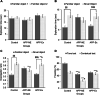
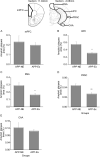
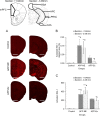
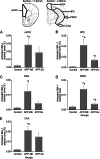
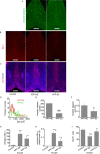
Results: The results showed that APPNL-G-F mice allowed to voluntarily exercise showed an improvement in cognitive functions. Furthermore, long-term exercise also improved anxiety in APPNL-G-F mice as assessed by measuring thigmotaxis in the Morris water task. We also found reductions in amyloid load and microgliosis, and a preservation of cholinergic cells in the brain of APPNL-G-F mice allowed to exercise in their home cages. These profound reductions in brain pathology associated with AD are likely responsible for the observed improvement of learning and memory functions following extensive and regular exercise.
Conclusion: These findings suggest the potential of physical exercise to mitigate the cognitive deficits in AD.
Keywords: APPNL-G-F mice; Alzheimer disease; Choline acetyltransferase; Cognitive dysfunction; Microgliosis; Physical exercise.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Repeated multi-domain cognitive training prevents cognitive decline, anxiety and amyloid pathology found in a mouse model of Alzheimer disease.Commun Biol. 2023 Nov 10;6(1):1145. doi: 10.1038/s42003-023-05506-6. Commun Biol. 2023. PMID: 37950055 Free PMC article.
-
Amyloid-β plaque formation and reactive gliosis are required for induction of cognitive deficits in App knock-in mouse models of Alzheimer's disease.BMC Neurosci. 2019 Mar 20;20(1):13. doi: 10.1186/s12868-019-0496-6. BMC Neurosci. 2019. PMID: 30894120 Free PMC article.
-
Age-dependent behavioral and biochemical characterization of single APP knock-in mouse (APPNL-G-F/NL-G-F) model of Alzheimer's disease.Neurobiol Aging. 2019 Mar;75:25-37. doi: 10.1016/j.neurobiolaging.2018.10.026. Epub 2018 Nov 5. Neurobiol Aging. 2019. PMID: 30508733
-
Cognitive and emotional alterations in App knock-in mouse models of Aβ amyloidosis.BMC Neurosci. 2018 Jul 28;19(1):46. doi: 10.1186/s12868-018-0446-8. BMC Neurosci. 2018. PMID: 30055565 Free PMC article.
-
Land/Water Aerobic Activities: Two Sides of the Same Coin. A Comparative Analysis on the Effects in Cognition of Alzheimer's Disease.J Alzheimers Dis. 2024;98(4):1181-1197. doi: 10.3233/JAD-231279. J Alzheimers Dis. 2024. PMID: 38552114 Review.
Cited by
-
Development and validation of prechiasmatic mouse model of subarachnoid hemorrhage to measure long-term neurobehavioral impairment.Res Sq [Preprint]. 2024 Apr 2:rs.3.rs-4176908. doi: 10.21203/rs.3.rs-4176908/v1. Res Sq. 2024. Update in: Adv Sci (Weinh). 2024 Dec;11(46):e2403977. doi: 10.1002/advs.202403977. PMID: 38645258 Free PMC article. Updated. Preprint.
-
Perinatal choline supplementation prevents learning and memory deficits and reduces brain amyloid Aβ42 deposition in AppNL-G-F Alzheimer's disease model mice.PLoS One. 2024 Feb 5;19(2):e0297289. doi: 10.1371/journal.pone.0297289. eCollection 2024. PLoS One. 2024. PMID: 38315685 Free PMC article.
-
Gut-brain axis: gut dysbiosis and psychiatric disorders in Alzheimer's and Parkinson's disease.Front Neurosci. 2023 Nov 13;17:1268419. doi: 10.3389/fnins.2023.1268419. eCollection 2023. Front Neurosci. 2023. PMID: 38075261 Free PMC article. Review.
-
Exposure to quasi-ultrafine particulate matter accelerates memory impairment and Alzheimer's disease-like neuropathology in the AppNL-G-F knock-in mouse model.Toxicol Sci. 2023 May 31;193(2):175-191. doi: 10.1093/toxsci/kfad036. Toxicol Sci. 2023. PMID: 37074955 Free PMC article.
-
Repeated multi-domain cognitive training prevents cognitive decline, anxiety and amyloid pathology found in a mouse model of Alzheimer disease.Commun Biol. 2023 Nov 10;6(1):1145. doi: 10.1038/s42003-023-05506-6. Commun Biol. 2023. PMID: 37950055 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

