Non-autonomous cell death induced by the Draper phagocytosis receptor requires signaling through the JNK and SRC pathways
- PMID: 36177600
- PMCID: PMC10658789
- DOI: 10.1242/jcs.250134
Non-autonomous cell death induced by the Draper phagocytosis receptor requires signaling through the JNK and SRC pathways
Abstract
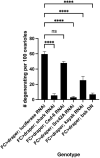
The last step of cell death is cell clearance, a process critical for tissue homeostasis. For efficient cell clearance to occur, phagocytes and dead cells need to reciprocally signal to each other. One important phenomenon that is under-investigated, however, is that phagocytes not only engulf corpses but contribute to cell death progression. The aims of this study were to determine how the phagocytic receptor Draper non-autonomously induces cell death, using the Drosophila ovary as a model system. We found that Draper, expressed in epithelial follicle cells, requires its intracellular signaling domain to kill the adjacent nurse cell population. Kinases Src42A, Shark and JNK (Bsk) were required for Draper-induced nurse cell death. Signs of nurse cell death occurred prior to apparent engulfment and required the caspase Dcp-1, indicating that it uses a similar apoptotic pathway to starvation-induced cell death. These findings indicate that active signaling by Draper is required to kill nurse cells via the caspase Dcp-1, providing novel insights into mechanisms of phagoptosis driven by non-professional phagocytes.
Keywords: Drosophila; Cell death; Draper; Ovary; Phagocyte; Phagoptosis.
© 2022. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures

Similar articles
-
Draper-dependent glial phagocytic activity is mediated by Src and Syk family kinase signalling.Nature. 2008 Jun 12;453(7197):935-9. doi: 10.1038/nature06901. Epub 2008 Apr 23. Nature. 2008. PMID: 18432193 Free PMC article.
-
Draper-mediated JNK signaling is required for glial phagocytosis of apoptotic neurons during Drosophila metamorphosis.Glia. 2018 Jul;66(7):1520-1532. doi: 10.1002/glia.23322. Epub 2018 Mar 9. Glia. 2018. PMID: 29520845
-
Draper acts through the JNK pathway to control synchronous engulfment of dying germline cells by follicular epithelial cells.Development. 2012 Nov;139(21):4029-39. doi: 10.1242/dev.082776. Epub 2012 Sep 19. Development. 2012. PMID: 22992958 Free PMC article.
-
Scrambled Eggs: Apoptotic Cell Clearance by Non-Professional Phagocytes in the Drosophila Ovary.Front Immunol. 2017 Nov 29;8:1642. doi: 10.3389/fimmu.2017.01642. eCollection 2017. Front Immunol. 2017. PMID: 29238344 Free PMC article. Review.
-
Distinct death mechanisms in Drosophila development.Curr Opin Cell Biol. 2010 Dec;22(6):889-95. doi: 10.1016/j.ceb.2010.08.022. Epub 2010 Sep 16. Curr Opin Cell Biol. 2010. PMID: 20846841 Free PMC article. Review.
Cited by
-
Cell death by phagocytosis.Nat Rev Immunol. 2024 Feb;24(2):91-102. doi: 10.1038/s41577-023-00921-6. Epub 2023 Aug 21. Nat Rev Immunol. 2024. PMID: 37604896 Review.
-
Activated Src kinase promotes cell cannibalism in Drosophila.J Cell Biol. 2023 Nov 6;222(11):e202302076. doi: 10.1083/jcb.202302076. Epub 2023 Sep 25. J Cell Biol. 2023. PMID: 37747450 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

