KIR-based inhibitory CARs overcome CAR-NK cell trogocytosis-mediated fratricide and tumor escape
- PMID: 36175679
- PMCID: PMC9942695
- DOI: 10.1038/s41591-022-02003-x
KIR-based inhibitory CARs overcome CAR-NK cell trogocytosis-mediated fratricide and tumor escape
Erratum in
-
Author Correction: KIR-based inhibitory CARs overcome CAR-NK cell trogocytosis-mediated fratricide and tumor escape.Nat Med. 2024 Mar;30(3):906. doi: 10.1038/s41591-023-02770-1. Nat Med. 2024. PMID: 38182787 No abstract available.
Abstract
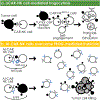
Trogocytosis is an active process that transfers surface material from targeted to effector cells. Using multiple in vivo tumor models and clinical data, we report that chimeric antigen receptor (CAR) activation in natural killer (NK) cells promoted transfer of the CAR cognate antigen from tumor to NK cells, resulting in (1) lower tumor antigen density, thus impairing the ability of CAR-NK cells to engage with their target, and (2) induced self-recognition and continuous CAR-mediated engagement, resulting in fratricide of trogocytic antigen-expressing NK cells (NKTROG+) and NK cell hyporesponsiveness. This phenomenon could be offset by a dual-CAR system incorporating both an activating CAR against the cognate tumor antigen and an NK self-recognizing inhibitory CAR that transferred a 'don't kill me' signal to NK cells upon engagement with their TROG+ siblings. This system prevented trogocytic antigen-mediated fratricide, while sparing activating CAR signaling against the tumor antigen, and resulted in enhanced CAR-NK cell activity.
© 2022. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
Disclosure of Conflict of Interest
RB, EL, LNK, PPB, SA, DM, MD, REC, EJS, KR, and The University of Texas MD Anderson Cancer Center have an institutional financial conflict of interest with Takeda Pharmaceutical. SA, DM, RB, EL, LNK, EJS, KR, and The University of Texas MD Anderson Cancer Center have an institutional financial conflict of interest with Affimed GmbH. KR participates on the Scientific Advisory Board for GemoAb, AvengeBio, Virogin Biotech, GSK, Caribou Biosciences, Navan Technologies and Bayer. The remaining authors declare no competing financial interests.
Figures





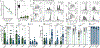

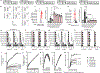







Similar articles
-
Outsmarting trogocytosis to boost CAR NK/T cell therapy.Mol Cancer. 2023 Nov 16;22(1):183. doi: 10.1186/s12943-023-01894-9. Mol Cancer. 2023. PMID: 37974170 Free PMC article. Review.
-
Trogocytosis in CAR immune cell therapy: a key mechanism of tumor immune escape.Cell Commun Signal. 2024 Oct 28;22(1):521. doi: 10.1186/s12964-024-01894-2. Cell Commun Signal. 2024. PMID: 39468646 Free PMC article. Review.
-
Challenges in αCD38-chimeric antigen receptor (CAR)-expressing natural killer (NK) cell-based immunotherapy in multiple myeloma: Harnessing the CD38dim phenotype of cytokine-stimulated NK cells as a strategy to prevent fratricide.Cytotherapy. 2023 Jul;25(7):763-772. doi: 10.1016/j.jcyt.2023.03.006. Epub 2023 Apr 11. Cytotherapy. 2023. PMID: 37055320
-
Bispecific antibody-mediated redirection of NKG2D-CAR natural killer cells facilitates dual targeting and enhances antitumor activity.J Immunother Cancer. 2021 Oct;9(10):e002980. doi: 10.1136/jitc-2021-002980. J Immunother Cancer. 2021. PMID: 34599028 Free PMC article.
-
CD19/CD20 dual-targeted chimeric antigen receptor-engineered natural killer cells exhibit improved cytotoxicity against acute lymphoblastic leukemia.J Transl Med. 2024 Mar 13;22(1):274. doi: 10.1186/s12967-024-04990-6. J Transl Med. 2024. PMID: 38475814 Free PMC article.
Cited by
-
Next-Generation CEA-CAR-NK-92 Cells against Solid Tumors: Overcoming Tumor Microenvironment Challenges in Colorectal Cancer.Cancers (Basel). 2024 Jan 16;16(2):388. doi: 10.3390/cancers16020388. Cancers (Basel). 2024. PMID: 38254876 Free PMC article.
-
CAR-T and CAR-NK as cellular cancer immunotherapy for solid tumors.Cell Mol Immunol. 2024 Oct;21(10):1089-1108. doi: 10.1038/s41423-024-01207-0. Epub 2024 Aug 12. Cell Mol Immunol. 2024. PMID: 39134804 Free PMC article. Review.
-
Targeting KIR as a novel approach to improve CAR-NK cell function.J Transl Genet Genom. 2023 Dec 5;7:230-235. doi: 10.20517/jtgg.2023.25. J Transl Genet Genom. 2023. PMID: 38229912 Free PMC article.
-
Advancements in CAR-NK therapy: lessons to be learned from CAR-T therapy.Front Immunol. 2023 May 2;14:1166038. doi: 10.3389/fimmu.2023.1166038. eCollection 2023. Front Immunol. 2023. PMID: 37205115 Free PMC article. Review.
-
Natural killer cell-related anti-tumour adoptive cell immunotherapy.J Cell Mol Med. 2024 Jun;28(11):e18362. doi: 10.1111/jcmm.18362. J Cell Mol Med. 2024. PMID: 38837666 Free PMC article. Review.
References
-
- Joly E & Hudrisier D What is trogocytosis and what is its purpose? Nat Immunol 4, 815 (2003). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

