Maturation of circulating Ly6ChiCCR2+ monocytes by mannan-MOG induces antigen-specific tolerance and reverses autoimmune encephalomyelitis
- PMID: 36159850
- PMCID: PMC9501702
- DOI: 10.3389/fimmu.2022.972003
Maturation of circulating Ly6ChiCCR2+ monocytes by mannan-MOG induces antigen-specific tolerance and reverses autoimmune encephalomyelitis
Abstract
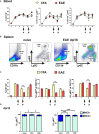
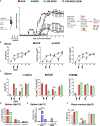
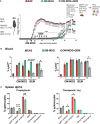
Autoimmune diseases affecting the CNS not only overcome immune privilege mechanisms that protect neural tissues but also peripheral immune tolerance mechanisms towards self. Together with antigen-specific T cells, myeloid cells are main effector cells in CNS autoimmune diseases such as multiple sclerosis, but the relative contributions of blood-derived monocytes and the tissue resident macrophages to pathology and repair is incompletely understood. Through the study of oxidized mannan-conjugated myelin oligodendrocyte glycoprotein 35-55 (OM-MOG), we show that peripheral maturation of Ly6ChiCCR2+ monocytes to Ly6ChiMHCII+PD-L1+ cells is sufficient to reverse spinal cord inflammation and demyelination in MOG-induced autoimmune encephalomyelitis. Soluble intradermal OM-MOG drains directly to the skin draining lymph node to be sequestered by subcapsular sinus macrophages, activates Ly6ChiCCR2+ monocytes to produce MHC class II and PD-L1, prevents immune cell trafficking to spinal cord, and reverses established lesions. We previously showed that protection by OM-peptides is antigen specific. Here, using a neutralizing anti-PD-L1 antibody in vivo and dendritic cell-specific Pdl1 knockout mice, we further demonstrate that PD-L1 in non-dendritic cells is essential for the therapeutic effects of OM-MOG. These results show that maturation of circulating Ly6ChiCCR2+ monocytes by OM-myelin peptides represents a novel mechanism of immune tolerance that reverses autoimmune encephalomyelitis.
Keywords: EAE; MDSC; PD-L1, Cre/loxP mouse; demyelination; immunotherapy; monocyte maturation; multiple sclerosis.
Copyright © 2022 Dagkonaki, Papalambrou, Avloniti, Gkika, Evangelidou, Androutsou, Tselios and Probert.
Conflict of interest statement
M-EA was employed by the company Vianex S.A. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
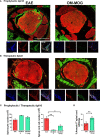
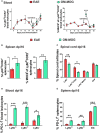
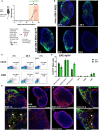
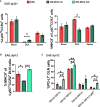
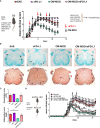
 PD-L1ff+OM-MOG versus dcPD-L1KO+OM-MOG), and one-way ANOVA followed by Tukey’s post hoc test (C) (*, #,
PD-L1ff+OM-MOG versus dcPD-L1KO+OM-MOG), and one-way ANOVA followed by Tukey’s post hoc test (C) (*, #,
 p ≤ 0.05, **, ## p ≤ 0.01).
p ≤ 0.05, **, ## p ≤ 0.01).Similar articles
-
Mannan-MOG35-55 Reverses Experimental Autoimmune Encephalomyelitis, Inducing a Peripheral Type 2 Myeloid Response, Reducing CNS Inflammation, and Preserving Axons in Spinal Cord Lesions.Front Immunol. 2020 Nov 19;11:575451. doi: 10.3389/fimmu.2020.575451. eCollection 2020. Front Immunol. 2020. PMID: 33329540 Free PMC article.
-
Mannan-conjugated myelin peptides prime non-pathogenic Th1 and Th17 cells and ameliorate experimental autoimmune encephalomyelitis.Exp Neurol. 2015 May;267:254-67. doi: 10.1016/j.expneurol.2014.10.019. Epub 2014 Oct 30. Exp Neurol. 2015. PMID: 25447934
-
Myelin Oligodendrocyte Glycoprotein (MOG)35-55 Mannan Conjugate Induces Human T-Cell Tolerance and Can Be Used as a Personalized Therapy for Multiple Sclerosis.Int J Mol Sci. 2024 May 31;25(11):6092. doi: 10.3390/ijms25116092. Int J Mol Sci. 2024. PMID: 38892275 Free PMC article.
-
T- and B-cell responses to myelin oligodendrocyte glycoprotein in experimental autoimmune encephalomyelitis and multiple sclerosis.Glia. 2001 Nov;36(2):220-34. doi: 10.1002/glia.1111. Glia. 2001. PMID: 11596130 Review.
-
The role of myelin oligodendrocyte glycoprotein in autoimmune demyelination: a target for multiple sclerosis therapy?Expert Opin Ther Targets. 2012 May;16(5):451-62. doi: 10.1517/14728222.2012.677438. Epub 2012 Apr 12. Expert Opin Ther Targets. 2012. PMID: 22494461 Review.
Cited by
-
Discrepant Phenotyping of Monocytes Based on CX3CR1 and CCR2 Using Fluorescent Reporters and Antibodies.Cells. 2024 May 10;13(10):819. doi: 10.3390/cells13100819. Cells. 2024. PMID: 38786041 Free PMC article.
-
IKKβ deletion from CNS macrophages increases neuronal excitability and accelerates the onset of EAE, while from peripheral macrophages reduces disease severity.J Neuroinflammation. 2024 Jan 27;21(1):34. doi: 10.1186/s12974-024-03023-9. J Neuroinflammation. 2024. PMID: 38279130 Free PMC article.
-
Spontaneous human CD8 T cell and autoimmune encephalomyelitis-induced CD4/CD8 T cell lesions in the brain and spinal cord of HLA-DRB1*15-positive multiple sclerosis humanized immune system mice.Elife. 2024 Jun 20;12:RP88826. doi: 10.7554/eLife.88826. Elife. 2024. PMID: 38900149 Free PMC article.
-
Myeloid-derived Suppressor Cells and Multiple Sclerosis.Curr Neuropharmacol. 2024;23(1):36-57. doi: 10.2174/1570159X22999240710142942. Curr Neuropharmacol. 2024. PMID: 38988152 Free PMC article. Review.
-
The Role of Myeloid-Derived Suppressor Cells in Multiple Sclerosis and Its Animal Model.Aging Dis. 2024 May 7;15(3):1329-1343. doi: 10.14336/AD.2023.0323-1. Aging Dis. 2024. PMID: 37307825 Free PMC article. Review.
References
-
- Ben-Nun A, Wekerle H, Cohen IR. Pillars article: The rapid isolation of clonable antigen-specific T lymphocyte lines capable of mediating autoimmune encephalomyelitis. J Immunol (2017) 198(9):3384–8. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

