PARK7 deficiency inhibits fatty acid β-oxidation via PTEN to delay liver regeneration after hepatectomy
- PMID: 36149763
- PMCID: PMC9505755
- DOI: 10.1002/ctm2.1061
PARK7 deficiency inhibits fatty acid β-oxidation via PTEN to delay liver regeneration after hepatectomy
Abstract
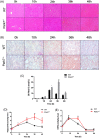
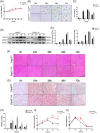
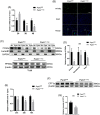
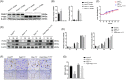
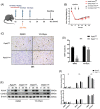
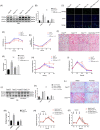
Transient regeneration-associated steatosis (TRAS) is a process of temporary hepatic lipid accumulation and is essential for liver regeneration by providing energy generated from fatty acid β-oxidation, but the regulatory mechanism underlying TRAS remains unknown. Parkinsonism-associated deglycase (Park7)/Dj1 is an important regulator involved in various liver diseases. In nonalcoholic fatty liver diseased mice, induced by a high-fat diet, Park7 deficiency improves hepatic steatosis, but its role in liver regeneration remains unknown METHODS: Park7 knockout (Park7-/- ), hepatocyte-specific Park7 knockout (Park7△hep ) and hepatocyte-specific Park7-Pten double knockout mice were subjected to 2/3 partial hepatectomy (PHx) RESULTS: Increased PARK7 expression was observed in the regenerating liver of mice at 36 and 48 h after PHx. Park7-/- and Park7△hep mice showed delayed liver regeneration and enhanced TRAS after PHx. PPARa, a key regulator of β-oxidation, and carnitine palmitoyltransferase 1a (CPT1a), a rate-limiting enzyme of β-oxidation, had substantially decreased expression in the regenerating liver of Park7△hep mice. Increased phosphatase and tensin homolog (PTEN) expression was observed in the liver of Park7△hep mice, which might contribute to delayed liver regeneration in these mice because genomic depletion or pharmacological inhibition of PTEN restored the delayed liver regeneration by reversing the downregulation of PPARa and CPT1a and in turn accelerating the utilization of TRAS in the regenerating liver of Park7△hep mice CONCLUSION: Park7/Dj1 is a novel regulator of PTEN-dependent fatty acid β-oxidation, and increasing Park7 expression might be a promising strategy to promote liver regeneration.
Keywords: Park7/Dj1; hepatocyte-specific knockout; liver regeneration; transient regeneration-associated steatosis.
© 2022 The Authors. Clinical and Translational Medicine published by John Wiley & Sons Australia, Ltd on behalf of Shanghai Institute of Clinical Bioinformatics.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this manuscript.
Figures


Similar articles
-
PTEN Down-Regulation Promotes β-Oxidation to Fuel Hypertrophic Liver Growth After Hepatectomy in Mice.Hepatology. 2017 Sep;66(3):908-921. doi: 10.1002/hep.29226. Epub 2017 Jul 20. Hepatology. 2017. PMID: 28437835
-
Hepatocyte Peroxisome Proliferator-Activated Receptor α Enhances Liver Regeneration after Partial Hepatectomy in Mice.Am J Pathol. 2019 Feb;189(2):272-282. doi: 10.1016/j.ajpath.2018.10.009. Epub 2018 Nov 16. Am J Pathol. 2019. PMID: 30448405 Free PMC article.
-
Delayed liver regeneration after partial hepatectomy in adiponectin knockout mice.Biochem Biophys Res Commun. 2009 Jan 2;378(1):68-72. doi: 10.1016/j.bbrc.2008.10.176. Epub 2008 Nov 12. Biochem Biophys Res Commun. 2009. PMID: 19013135
-
Lipid droplet deposition in the regenerating liver: A promoter, inhibitor, or bystander?Hepatol Commun. 2023 Sep 15;7(10):e0267. doi: 10.1097/HC9.0000000000000267. eCollection 2023 Oct 1. Hepatol Commun. 2023. PMID: 37708445 Free PMC article. Review.
-
Key hepatoprotective roles of mitochondria in liver regeneration.Am J Physiol Gastrointest Liver Physiol. 2023 Mar 1;324(3):G207-G218. doi: 10.1152/ajpgi.00220.2022. Epub 2023 Jan 17. Am J Physiol Gastrointest Liver Physiol. 2023. PMID: 36648139 Free PMC article. Review.
Cited by
-
Integrative analysis of the transcriptome and metabolome reveals the importance of hepatokine FGF21 in liver aging.Genes Dis. 2023 Nov 7;11(5):101161. doi: 10.1016/j.gendis.2023.101161. eCollection 2024 Sep. Genes Dis. 2023. PMID: 39022127 Free PMC article.
-
Liver Extracellular Vesicles and Particles Enriched β-Sitosterol Effectively Promote Liver Regeneration in Mice.Int J Nanomedicine. 2024 Aug 8;19:8117-8137. doi: 10.2147/IJN.S465346. eCollection 2024. Int J Nanomedicine. 2024. PMID: 39139504 Free PMC article.
-
Transcriptomic Characterization of Key Factors and Signaling Pathways for the Regeneration of Partially Hepatectomized Liver in Zebrafish.Int J Mol Sci. 2024 Jun 29;25(13):7212. doi: 10.3390/ijms25137212. Int J Mol Sci. 2024. PMID: 39000319 Free PMC article.
-
Unsaturated or saturated dietary fat-mediated steatosis impairs hepatic regeneration following partial hepatectomy in mice.PLoS One. 2023 May 11;18(5):e0284428. doi: 10.1371/journal.pone.0284428. eCollection 2023. PLoS One. 2023. PMID: 37167305 Free PMC article.
-
Effect of Hepatic Lipid Overload on Accelerated Hepatocyte Proliferation Promoted by HGF Expression via the SphK1/S1PR2 Pathway in MCD-diet Mouse Partial Hepatectomy.Acta Histochem Cytochem. 2024 Oct 28;57(5):175-188. doi: 10.1267/ahc.24-00046. Epub 2024 Oct 23. Acta Histochem Cytochem. 2024. PMID: 39552932 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
