Genome integrity and inflammation in the nervous system
- PMID: 36148701
- PMCID: PMC9844216
- DOI: 10.1016/j.dnarep.2022.103406
Genome integrity and inflammation in the nervous system
Abstract
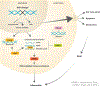
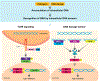
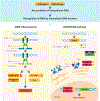
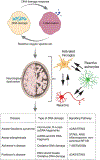
Preservation of genomic integrity is crucial for nervous system development and function. DNA repair deficiency results in several human diseases that are characterized by both neurodegeneration and neuroinflammation. Recent research has highlighted a role for compromised genomic integrity as a key factor driving neuropathology and triggering innate immune signaling to cause inflammation. Here we review the mechanisms by which DNA damage engages innate immune signaling and how this may promote neurological disease. We also consider the contributions of different neural cell types towards DNA damage-driven neuroinflammation. A deeper knowledge of genome maintenance mechanisms that prevent aberrant immune activation in neural cells will guide future therapies to ameliorate neurological disease.
Keywords: Astrocyte; DNA damage; Microglia; Nervous system; Neuroinflammation.
Copyright © 2022 Elsevier B.V. All rights reserved.
Figures
Similar articles
-
Maintaining genome stability in the nervous system.Nat Neurosci. 2013 Nov;16(11):1523-9. doi: 10.1038/nn.3537. Epub 2013 Oct 28. Nat Neurosci. 2013. PMID: 24165679 Free PMC article. Review.
-
Genome integrity and disease prevention in the nervous system.Genes Dev. 2017 Jun 15;31(12):1180-1194. doi: 10.1101/gad.301325.117. Genes Dev. 2017. PMID: 28765160 Free PMC article. Review.
-
Microglial inflammation in genome instability: A neurodegenerative perspective.DNA Repair (Amst). 2024 Mar;135:103634. doi: 10.1016/j.dnarep.2024.103634. Epub 2024 Jan 24. DNA Repair (Amst). 2024. PMID: 38290197
-
Responding to DNA double strand breaks in the nervous system.Neuroscience. 2007 Apr 14;145(4):1365-74. doi: 10.1016/j.neuroscience.2006.07.026. Epub 2006 Aug 23. Neuroscience. 2007. PMID: 16934412 Review.
-
Neuronal injury in chronic CNS inflammation.Best Pract Res Clin Anaesthesiol. 2010 Dec;24(4):551-62. doi: 10.1016/j.bpa.2010.11.001. Epub 2010 Nov 29. Best Pract Res Clin Anaesthesiol. 2010. PMID: 21619866 Review.
Cited by
-
YIPF2 regulates genome integrity.Cell Biosci. 2024 Sep 5;14(1):114. doi: 10.1186/s13578-024-01300-x. Cell Biosci. 2024. PMID: 39238039 Free PMC article.
-
Introducing the Role of Genotoxicity in Neurodegenerative Diseases and Neuropsychiatric Disorders.Int J Mol Sci. 2024 Jun 29;25(13):7221. doi: 10.3390/ijms25137221. Int J Mol Sci. 2024. PMID: 39000326 Free PMC article. Review.
-
cGAS-STING signalling regulates microglial chemotaxis in genome instability.Nucleic Acids Res. 2024 Feb 9;52(3):1188-1206. doi: 10.1093/nar/gkad1184. Nucleic Acids Res. 2024. PMID: 38084916 Free PMC article.
-
The missing hallmark of health: psychosocial adaptation.Cell Stress. 2024 Mar 12;8:21-50. doi: 10.15698/cst2024.03.294. eCollection 2024. Cell Stress. 2024. PMID: 38476764 Free PMC article.
-
DNA damage and repair: underlying mechanisms leading to microcephaly.Front Cell Dev Biol. 2023 Oct 10;11:1268565. doi: 10.3389/fcell.2023.1268565. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37881689 Free PMC article. Review.
References
-
- Soriani A, Zingoni A, Cerboni C, Iannitto ML, Ricciardi MR, Di Gialleonardo V, Cippitelli M, Fionda C, Petrucci MT, Guarini A, Foa R, Santoni A, ATM-ATR-dependent upregulation of DNAM-1 and NKG2D ligands on multiple myeloma cells by therapeutic agents results in enhanced NK-cell susceptibility and is associated with a senescent phenotype, Blood, 113 (2009) 3503–3511. - PubMed
-
- Ardolino M, Zingoni A, Cerboni C, Cecere F, Soriani A, Iannitto ML, Santoni A, DNAM-1 ligand expression on Ag-stimulated T lymphocytes is mediated by ROS-dependent activation of DNA-damage response: relevance for NK-T cell interaction, Blood, 117 (2011) 4778–4786. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

