Characterization of Transcriptional Responses to Genomovirus Infection of the White Mold Fungus, Sclerotinia sclerotiorum
- PMID: 36146699
- PMCID: PMC9506476
- DOI: 10.3390/v14091892
Characterization of Transcriptional Responses to Genomovirus Infection of the White Mold Fungus, Sclerotinia sclerotiorum
Abstract
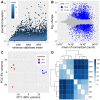
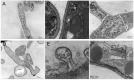
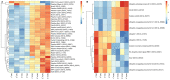
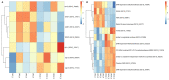
Soybean leaf-associated gemygorvirus-1 (SlaGemV-1) is a CRESS-DNA virus classified in the family Genomoviridae, which causes hypovirulence and abolishes sclerotia formation in infected fungal pathogens under the family Sclerotiniaceae. To investigate the mechanisms involved in the induction of hypovirulence, RNA-Seq was compared between virus-free and SlaGemV-1-infected Sclerotinia sclerotiorum strain DK3. Overall, 4639 genes were differentially expressed, with 50.5% up regulated and 49.5% down regulated genes. GO enrichments suggest changes in integral membrane components and transmission electron microscopy images reveal virus-like particles localized near the inner cell membrane. Differential gene expression analysis focused on genes responsible for cell cycle and DNA replication and repair pathways, ubiquitin proteolysis, gene silencing, methylation, pathogenesis-related, sclerotial development, carbohydrate metabolism, and oxalic acid biosynthesis. Carbohydrate metabolism showed the most changes, with two glycoside hydrolase genes being the most down regulated by -2396.1- and -648.6-fold. Genes relating to pathogenesis showed consistent down regulation with the greatest being SsNep1, SsSSVP1, and Endo2 showing, -4555-, -14.7-, and -12.3-fold changes. The cell cycle and DNA replication/repair pathways were almost entirely up regulated including a putative cyclin and separase being up regulated 8.3- and 5.2-fold. The oxalate decarboxylase genes necessary for oxalic acid catabolism and oxalic acid precursor biosynthesis genes and its metabolism show down regulations of -17.2- and -12.1-fold changes. Sclerotial formation genes also appear differentially regulated including a melanin biosynthesis gene Pks1 and a sclerotia formation gene Sl2 with fold changes of 3.8 and -2.9.
Keywords: RNA-Seq; Sclerotinia sclerotiorum; genomovirus; hypovirulence; mycovirus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Mycovirus-induced hypovirulence in notorious fungi Sclerotinia: a comprehensive review.Braz J Microbiol. 2023 Sep;54(3):1459-1478. doi: 10.1007/s42770-023-01073-4. Epub 2023 Jul 31. Braz J Microbiol. 2023. PMID: 37523037 Free PMC article. Review.
-
Identification of the Viral Determinant of Hypovirulence and Host Range in Sclerotiniaceae of a Genomovirus Reconstructed from the Plant Metagenome.J Virol. 2021 Aug 10;95(17):e0026421. doi: 10.1128/JVI.00264-21. Epub 2021 Aug 10. J Virol. 2021. PMID: 34132570 Free PMC article.
-
Transfection of Sclerotinia sclerotiorum with in vitro transcripts of a naturally occurring interspecific recombinant of Sclerotinia sclerotiorum hypovirus 2 significantly reduces virulence of the fungus.J Virol. 2015 May;89(9):5060-71. doi: 10.1128/JVI.03199-14. Epub 2015 Feb 18. J Virol. 2015. PMID: 25694604 Free PMC article.
-
A Sclerotinia sclerotiorum Transcription Factor Involved in Sclerotial Development and Virulence on Pea.mSphere. 2019 Jan 23;4(1):e00615-18. doi: 10.1128/mSphere.00615-18. mSphere. 2019. PMID: 30674647 Free PMC article.
-
Sclerotinia sclerotiorum: An Evaluation of Virulence Theories.Annu Rev Phytopathol. 2018 Aug 25;56:311-338. doi: 10.1146/annurev-phyto-080417-050052. Epub 2018 Jun 29. Annu Rev Phytopathol. 2018. PMID: 29958073 Review.
Cited by
-
Experimental verification of strain-dependent relationship between mycovirus and its fungal host.iScience. 2023 Jul 10;26(8):107337. doi: 10.1016/j.isci.2023.107337. eCollection 2023 Aug 18. iScience. 2023. PMID: 37520716 Free PMC article.
-
Mycovirus-induced hypovirulence in notorious fungi Sclerotinia: a comprehensive review.Braz J Microbiol. 2023 Sep;54(3):1459-1478. doi: 10.1007/s42770-023-01073-4. Epub 2023 Jul 31. Braz J Microbiol. 2023. PMID: 37523037 Free PMC article. Review.
References
-
- Peltier A.J., Bradley C.A., Chilvers M.I., Malvick D.K., Mueller D.S., Wise K.A., Esker P.D. Biology, Yield loss and Control of Sclerotinia Stem Rot of Soybean. J. Integr. Pest Manag. 2012;3:B1–B7. doi: 10.1603/IPM11033. - DOI
-
- Nuss D.L. Mycoviruses. Cell. Mol. Biol. Filam. Fungi. 2010:145–152. doi: 10.1128/9781555816636.ch12. - DOI
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources

