Revisiting Cerebrospinal Fluid Flow Direction and Rate in Physiologically Based Pharmacokinetic Model
- PMID: 36145511
- PMCID: PMC9504371
- DOI: 10.3390/pharmaceutics14091764
Revisiting Cerebrospinal Fluid Flow Direction and Rate in Physiologically Based Pharmacokinetic Model
Abstract
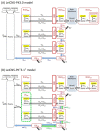
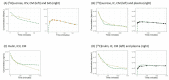
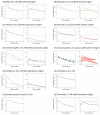
The bidirectional pulsatile movement of cerebrospinal fluid (CSF), instead of the traditionally believed unidirectional and constant CSF circulation, has been demonstrated. In the present study, the structure and parameters of the CSF compartments were revisited in our comprehensive and validated central nervous system (CNS)-specific, physiologically based pharmacokinetic (PBPK) model of healthy rats (LeiCNS-PK3.0). The bidirectional and site-dependent CSF movement was incorporated into LeiCNS-PK3.0 to create the new LeiCNS-PK"3.1" model. The physiological CSF movement rates in healthy rats that are unavailable from the literature were estimated by fitting the PK data of sucrose, a CSF flow marker, after intra-CSF administration. The capability of LeiCNS-PK3.1 to describe the PK profiles of other molecules was compared with that of the original LeiCNS-PK3.0 model. LeiCNS-PK3.1 demonstrated superior description of the CSF PK profiles of a range of small molecules after intra-CSF administration over LeiCNS-PK3.0. LeiCNS-PK3.1 also retained the same level of predictability of CSF PK profiles in cisterna magna after intravenous administration. These results support the theory of bidirectional and site-dependent CSF movement across the entire CSF space over unidirectional and constant CSF circulation in healthy rats, pointing out the need to revisit the structures and parameters of CSF compartments in CNS-PBPK models.
Keywords: CSF; CSF physiology; bidirectional pulsatile CSF movement; cerebrospinal fluid; intra-CSF administration; physiologically based pharmacokinetic model.
Conflict of interest statement
M.H. is an employee of Daiichi-Sankyo Co., Ltd. The other author declare no conflict of interest.
Figures

Similar articles
-
Lumbar cerebrospinal fluid-to-brain extracellular fluid surrogacy is context-specific: insights from LeiCNS-PK3.0 simulations.J Pharmacokinet Pharmacodyn. 2021 Oct;48(5):725-741. doi: 10.1007/s10928-021-09768-7. Epub 2021 Jun 17. J Pharmacokinet Pharmacodyn. 2021. PMID: 34142308 Free PMC article.
-
Using the LeiCNS-PK3.0 Physiologically-Based Pharmacokinetic Model to Predict Brain Extracellular Fluid Pharmacokinetics in Mice.Pharm Res. 2023 Nov;40(11):2555-2566. doi: 10.1007/s11095-023-03554-5. Epub 2023 Jul 13. Pharm Res. 2023. PMID: 37442882 Free PMC article.
-
Drug Distribution in Brain and Cerebrospinal Fluids in Relation to IC50 Values in Aging and Alzheimer's Disease, Using the Physiologically Based LeiCNS-PK3.0 Model.Pharm Res. 2022 Jul;39(7):1303-1319. doi: 10.1007/s11095-022-03281-3. Epub 2022 May 23. Pharm Res. 2022. PMID: 35606598 Free PMC article.
-
A new look at cerebrospinal fluid circulation.Fluids Barriers CNS. 2014 May 1;11:10. doi: 10.1186/2045-8118-11-10. eCollection 2014. Fluids Barriers CNS. 2014. PMID: 24817998 Free PMC article. Review.
-
Physiologically based pharmacokinetic modeling to investigate regional brain distribution kinetics in rats.AAPS J. 2012 Sep;14(3):543-53. doi: 10.1208/s12248-012-9366-1. Epub 2012 May 17. AAPS J. 2012. PMID: 22588644 Free PMC article. Review.
Cited by
-
In Silico Pharmacology for Evidence-Based and Precision Medicine.Pharmaceutics. 2023 Mar 22;15(3):1014. doi: 10.3390/pharmaceutics15031014. Pharmaceutics. 2023. PMID: 36986874 Free PMC article.
-
Mathematical modeling of transdermal delivery of topical drug formulations in a dynamic microfluidic diffusion chamber in health and disease.PLoS One. 2024 Apr 11;19(4):e0299501. doi: 10.1371/journal.pone.0299501. eCollection 2024. PLoS One. 2024. PMID: 38603673 Free PMC article.
-
The relationship between myodural bridge, atrophy and hyperplasia of the suboccipital musculature, and cerebrospinal fluid dynamics.Sci Rep. 2023 Nov 2;13(1):18882. doi: 10.1038/s41598-023-45820-x. Sci Rep. 2023. PMID: 37919345 Free PMC article.
-
Epidural pressure measurement using a fiber-optic sensor (proof-of-principle in vivo animal trial).Animal Model Exp Med. 2024 Oct;7(5):769-776. doi: 10.1002/ame2.12469. Epub 2024 Jul 9. Animal Model Exp Med. 2024. PMID: 38981680 Free PMC article.
-
Interspecies Brain PBPK Modeling Platform to Predict Passive Transport through the Blood-Brain Barrier and Assess Target Site Disposition.Pharmaceutics. 2024 Feb 4;16(2):226. doi: 10.3390/pharmaceutics16020226. Pharmaceutics. 2024. PMID: 38399280 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

