Grifola frondosa Extract Containing Bioactive Components Blocks Skin Fibroblastic Inflammation and Cytotoxicity Caused by Endocrine Disrupting Chemical, Bisphenol A
- PMID: 36145189
- PMCID: PMC9503552
- DOI: 10.3390/nu14183812
Grifola frondosa Extract Containing Bioactive Components Blocks Skin Fibroblastic Inflammation and Cytotoxicity Caused by Endocrine Disrupting Chemical, Bisphenol A
Abstract
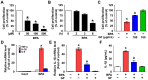
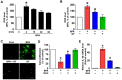
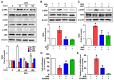
Grifola frondosa (GF), a species of Basidiomycotina, is widely distributed across Asia and has been used as an immunomodulatory, anti-bacterial, and anti-cancer agent. In the present study, the pharmacological activity of the GF extract against an ecotoxicological industrial chemical, bisphenol A (BPA) in normal human dermal fibroblasts (NHDFs), was investigated. GF extract containing naringin, hesperidin, chlorogenic acid, and kaempferol showed an inhibitory effect on cell death and inflammation induced by BPA in the NHDFs. For the cell death caused by BPA, GF extract inhibited the production of reactive oxygen species responsible for the unique activation of the extracellular signal-regulated kinase. In addition, GF extract attenuated the expression of apoptosis-related proteins (Bax, Bcl-2, and cleaved caspase-3) and the pro-inflammatory cytokine IL-1β by the suppression of the redox-sensitive transcription factor, nuclear factor-kappa B (NF-κB) in BPA-treated NHDFs. For the inflammation triggered by BPA, GF extract blocked the inflammasome-mediated caspase-1 activation that leads to the secretion of IL-1β protein. These results indicate that the GF extract is a functional antioxidant that prevents skin fibroblastic pyroptosis induced by BPA.
Keywords: Grifola frondosa; apoptotic cell death; bisphenol A; normal human dermal fibroblasts; pyroptosis; reactive oxygen species.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Unravelling bisphenol A-induced hepatotoxicity: Insights into oxidative stress, inflammation, and energy dysregulation.Environ Pollut. 2024 Dec 1;362:124922. doi: 10.1016/j.envpol.2024.124922. Epub 2024 Sep 12. Environ Pollut. 2024. PMID: 39260547
-
Immunomodulatory activities of medicinal mushroom Grifola frondosa extract and its bioactive constituent.Am J Chin Med. 2013;41(1):131-44. doi: 10.1142/S0192415X13500109. Am J Chin Med. 2013. PMID: 23336512
-
Kaempferol Blocks the Skin Fibroblastic Interleukin 1β Expression and Cytotoxicity Induced by 12-O-tetradecanoylphorbol-13-acetate by Suppressing c-Jun N-terminal Kinase.Nutrients. 2021 Sep 1;13(9):3079. doi: 10.3390/nu13093079. Nutrients. 2021. PMID: 34578957 Free PMC article.
-
Grifola frondosa polysaccharides induce breast cancer cell apoptosis via the mitochondrial-dependent apoptotic pathway.Int J Mol Med. 2017 Oct;40(4):1089-1095. doi: 10.3892/ijmm.2017.3081. Epub 2017 Jul 26. Int J Mol Med. 2017. PMID: 28765878 Free PMC article.
-
Antitumor, Anti-Inflammatory and Antiallergic Effects of Agaricus blazei Mushroom Extract and the Related Medicinal Basidiomycetes Mushrooms, Hericium erinaceus and Grifola frondosa: A Review of Preclinical and Clinical Studies.Nutrients. 2020 May 8;12(5):1339. doi: 10.3390/nu12051339. Nutrients. 2020. PMID: 32397163 Free PMC article. Review.
Cited by
-
Antioxidant and Immunomodulatory Activities of Polysaccharides from Fermented Wheat Products of Grifola frondosa: In Vitro Methods.Int J Food Sci. 2023 Aug 9;2023:3820276. doi: 10.1155/2023/3820276. eCollection 2023. Int J Food Sci. 2023. PMID: 37593692 Free PMC article.
-
Occurrence, efficiency of treatment processes, source apportionment and human health risk assessment of pharmaceuticals and xenoestrogen compounds in tap water from some Ghanaian communities.Heliyon. 2024 May 23;10(11):e31815. doi: 10.1016/j.heliyon.2024.e31815. eCollection 2024 Jun 15. Heliyon. 2024. PMID: 38845891 Free PMC article.
-
Extracts of Grifola frondosa inhibit the MAPK signaling pathways involved in keratinocyte inflammation and ameliorate atopic dermatitis.Nutr Res Pract. 2023 Dec;17(6):1056-1069. doi: 10.4162/nrp.2023.17.6.1056. Epub 2023 Nov 15. Nutr Res Pract. 2023. PMID: 38053833 Free PMC article.
-
Unveiling the full spectrum of maitake mushrooms: A comprehensive review of their medicinal, therapeutic, nutraceutical, and cosmetic potential.Heliyon. 2024 Apr 26;10(9):e30254. doi: 10.1016/j.heliyon.2024.e30254. eCollection 2024 May 15. Heliyon. 2024. PMID: 38707308 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

