Chemical and Antimicrobial Characterization of Mentha piperita L. and Rosmarinus officinalis L. Essential Oils and In Vitro Potential Cytotoxic Effect in Human Colorectal Carcinoma Cells
- PMID: 36144839
- PMCID: PMC9505364
- DOI: 10.3390/molecules27186106
Chemical and Antimicrobial Characterization of Mentha piperita L. and Rosmarinus officinalis L. Essential Oils and In Vitro Potential Cytotoxic Effect in Human Colorectal Carcinoma Cells
Abstract
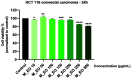
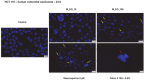
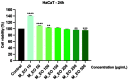
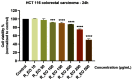
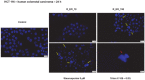
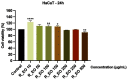
Colorectal cancer is one of the most frequently diagnosed forms of cancer, and the therapeutic solutions are frequently aggressive requiring improvements. Essential oils (EOs) are secondary metabolites of aromatic plants with important pharmacological properties that proved to be beneficial in multiple pathologies including cancer. Mentha piperita L. (M_EO) and Rosmarinus officinalis L. (R_EO) essential oils are well-known for their biological effects (antimicrobial, antioxidant, anti-inflammatory and cytotoxic in different cancer cells), but their potential as complementary treatment in colorectal cancer is underexplored. The aim of the present study was to investigate the M_EO and R_EO in terms of chemical composition, antioxidant, antimicrobial, and cytotoxic effects in a colorectal cancer cell line-HCT 116. The gas-chromatographic analysis revealed menthone and menthol, and eucalyptol, α-pinene and L-camphor as major compounds in M_EO and R_EO respectively. M_EO exhibited potent antimicrobial activity, moderate antioxidant activity and a low cytotoxic effect in HCT 116 cells. R_EO presented a significant cytotoxicity in colorectal cancer cells and a low antimicrobial effect. The cytotoxic effect on non-cancerous cell line HaCaT was not significant for both essential oils. These results may provide an experimental basis for further research concerning the potential use of M_EO and R_EO for anticancer treatment.
Keywords: Mentha piperita L. essential oil; Rosmarinus officinalis L. essential oil; antimicrobial potential; colorectal cancer; cytotoxicity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Mentha piperita: Essential Oil and Extracts, Their Biological Activities, and Perspectives on the Development of New Medicinal and Cosmetic Products.Molecules. 2023 Nov 6;28(21):7444. doi: 10.3390/molecules28217444. Molecules. 2023. PMID: 37959863 Free PMC article. Review.
-
Chemical composition of Mentha pulegium and Rosmarinus officinalis essential oils and their antileishmanial, antibacterial and antioxidant activities.Microb Pathog. 2017 Oct;111:41-49. doi: 10.1016/j.micpath.2017.08.015. Epub 2017 Aug 15. Microb Pathog. 2017. PMID: 28821401
-
Phytochemical Analysis and Antioxidant and Antifungal Activities of Powders, Methanol Extracts, and Essential Oils from Rosmarinus officinalis L. and Thymus ciliatus Desf. Benth.Int J Mol Sci. 2024 Jul 22;25(14):7989. doi: 10.3390/ijms25147989. Int J Mol Sci. 2024. PMID: 39063231 Free PMC article.
-
Chemical Composition and in vivo Efficacy of the Essential Oil of Mentha piperita L. in the Suppression of Crown Gall Disease on Tomato Plants.J Oleo Sci. 2019 May 1;68(5):419-426. doi: 10.5650/jos.ess18261. Epub 2019 Mar 13. J Oleo Sci. 2019. PMID: 30867394
-
Agrobiological Interactions of Essential Oils of Two Menthol Mints: Mentha piperita and Mentha arvensis.Molecules. 2019 Dec 23;25(1):59. doi: 10.3390/molecules25010059. Molecules. 2019. PMID: 31878007 Free PMC article. Review.
Cited by
-
Antimicrobial Effects of Edible Mixed Herbal Extracts on Oral Microorganisms: An In Vitro Study.Medicina (Kaunas). 2023 Oct 4;59(10):1771. doi: 10.3390/medicina59101771. Medicina (Kaunas). 2023. PMID: 37893489 Free PMC article.
-
Mentha piperita: Essential Oil and Extracts, Their Biological Activities, and Perspectives on the Development of New Medicinal and Cosmetic Products.Molecules. 2023 Nov 6;28(21):7444. doi: 10.3390/molecules28217444. Molecules. 2023. PMID: 37959863 Free PMC article. Review.
-
Comparative Cytotoxicity of Menthol and Eucalyptol: An In Vitro Study on Human Gingival Fibroblasts.Pharmaceutics. 2024 Apr 9;16(4):521. doi: 10.3390/pharmaceutics16040521. Pharmaceutics. 2024. PMID: 38675182 Free PMC article.
-
Potential Use and Chemical Analysis of Some Natural Plant Extracts for Controlling Listeria spp. Growth In Vitro and in Food.Foods. 2024 Sep 14;13(18):2915. doi: 10.3390/foods13182915. Foods. 2024. PMID: 39335846 Free PMC article.
-
Phytochemical Variability, In Vitro and In Vivo Biological Investigations, and In Silico Antibacterial Mechanisms of Mentha piperita Essential Oils Collected from Two Different Regions in Morocco.Foods. 2022 Nov 1;11(21):3466. doi: 10.3390/foods11213466. Foods. 2022. PMID: 36360079 Free PMC article.
References
-
- Spisni E., Petrocelli G., Imbesi V., Spigarelli R., Azzinnari D., Donati Sarti M., Campieri M., Valerii M.C. Antioxidant, An-ti-Inflammatory, and Microbial-Modulating Activities of Essential Oils: Implications in Colonic Pathophysiology. Int. J. Mol. Sci. 2020;21:4152. doi: 10.3390/ijms21114152. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

