Do Bacteria Provide an Alternative to Cancer Treatment and What Role Does Lactic Acid Bacteria Play?
- PMID: 36144335
- PMCID: PMC9501580
- DOI: 10.3390/microorganisms10091733
Do Bacteria Provide an Alternative to Cancer Treatment and What Role Does Lactic Acid Bacteria Play?
Abstract
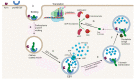
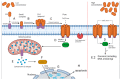
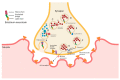
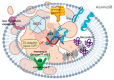
Cancer is one of the leading causes of mortality and morbidity worldwide. According to 2022 statistics from the World Health Organization (WHO), close to 10 million deaths have been reported in 2020 and it is estimated that the number of cancer cases world-wide could increase to 21.6 million by 2030. Breast, lung, thyroid, pancreatic, liver, prostate, bladder, kidney, pelvis, colon, and rectum cancers are the most prevalent. Each year, approximately 400,000 children develop cancer. Treatment between countries vary, but usually includes either surgery, radiotherapy, or chemotherapy. Modern treatments such as hormone-, immuno- and antibody-based therapies are becoming increasingly popular. Several recent reports have been published on toxins, antibiotics, bacteriocins, non-ribosomal peptides, polyketides, phenylpropanoids, phenylflavonoids, purine nucleosides, short chain fatty acids (SCFAs) and enzymes with anticancer properties. Most of these molecules target cancer cells in a selective manner, either directly or indirectly through specific pathways. This review discusses the role of bacteria, including lactic acid bacteria, and their metabolites in the treatment of cancer.
Keywords: bacteria; cancer treatment; lactic acid bacteria.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Bacteria and bacterial anticancer agents as a promising alternative for cancer therapeutics.Biochimie. 2020 Oct;177:164-189. doi: 10.1016/j.biochi.2020.07.020. Epub 2020 Aug 19. Biochimie. 2020. PMID: 32827604 Review.
-
Estimates of cancer incidence and mortality in Europe in 1995.Eur J Cancer. 2002 Jan;38(1):99-166. doi: 10.1016/s0959-8049(01)00350-1. Eur J Cancer. 2002. PMID: 11750846
-
Overview of resistance to systemic therapy in patients with breast cancer.Adv Exp Med Biol. 2007;608:1-22. doi: 10.1007/978-0-387-74039-3_1. Adv Exp Med Biol. 2007. PMID: 17993229 Review.
-
Italian cancer figures, report 2012: Cancer in children and adolescents.Epidemiol Prev. 2013 Jan-Feb;37(1 Suppl 1):1-225. Epidemiol Prev. 2013. PMID: 23585445 English, Italian.
-
More advantages in detecting bone and soft tissue metastases from prostate cancer using 18F-PSMA PET/CT.Hell J Nucl Med. 2019 Jan-Apr;22(1):6-9. doi: 10.1967/s002449910952. Epub 2019 Mar 7. Hell J Nucl Med. 2019. PMID: 30843003
Cited by
-
Cytotoxic activity and apoptosis induction by supernatant of Lentilactobacillus buchneri on HT-29 colon cancer cells.Iran J Microbiol. 2024 Apr;16(2):219-226. doi: 10.18502/ijm.v16i2.15355. Iran J Microbiol. 2024. PMID: 38854985 Free PMC article.
-
Advancements in the impact of human microbiota and probiotics on leukemia.Front Microbiol. 2024 Jul 3;15:1423838. doi: 10.3389/fmicb.2024.1423838. eCollection 2024. Front Microbiol. 2024. PMID: 39021626 Free PMC article. Review.
-
Probiotic-Derived Bioactive Compounds in Colorectal Cancer Treatment.Microorganisms. 2023 Jul 27;11(8):1898. doi: 10.3390/microorganisms11081898. Microorganisms. 2023. PMID: 37630458 Free PMC article. Review.
-
Gut Bacteria Provide Genetic and Molecular Reporter Systems to Identify Specific Diseases.Int J Mol Sci. 2024 Apr 17;25(8):4431. doi: 10.3390/ijms25084431. Int J Mol Sci. 2024. PMID: 38674014 Free PMC article. Review.
References
-
- World Health Organization World Health Assembly, 70 Cancer Prevention and Control in the Context of an Integrated Approach. [(accessed on 21 June 2022)]. Available online: https://apps.who.int/iris/handle/10665/275676.
-
- National Institute for Communicable Diseases National Cancer Registry 2019, Full Report. [(accessed on 21 June 2022)]. Available online: https://www.nicd.ac.za/centres/national-cancer-registry/cancer-statistics.
-
- American Cancer Society, Cancer Facts and Figures. 2018. [(accessed on 20 June 2022)]. Available online: - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

