Biological Evaluation of 3-Azaspiro[Bicyclo[3.1.0]Hexane-2,5'-Pyrimidines] as Potential Antitumor Agents
- PMID: 36142688
- PMCID: PMC9506420
- DOI: 10.3390/ijms231810759
Biological Evaluation of 3-Azaspiro[Bicyclo[3.1.0]Hexane-2,5'-Pyrimidines] as Potential Antitumor Agents
Abstract
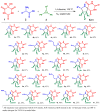
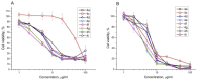
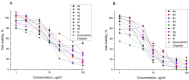
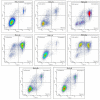
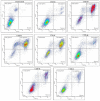
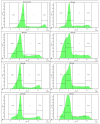
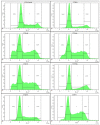
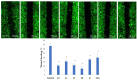
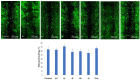
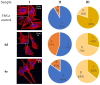
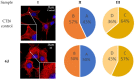
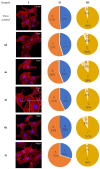
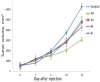
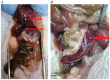
A series of heterocyclic compounds containing spirofused barbiturate and 3-azabicyclo[3.1.0]hexane frameworks have been studied as potential antitumor agents. Antiproliferative activity of products was screened in human erythroleukemia (K562), T lymphocyte (Jurkat), and cervical carcinoma (HeLa) as well as mouse colon carcinoma (CT26) and African green monkey kidney epithelial (Vero) cell lines. The most effective among the screened compounds show IC50 in the range from 4.2 to 24.1 μM for all tested cell lines. The screened compounds have demonstrated a significant effect of the distribution of HeLa and CT26 cells across the cell cycle stage, with accumulation of cells in SubG1 phase and induced apoptosis. It was found, using a confocal microscopy, that actin filaments disappeared and granular actin was distributed diffusely in the cytoplasm of up to 90% of HeLa cells and up to 64% of CT26 cells after treatment with tested 3-azaspiro[bicyclo [3.1.0]hexane-2,5'-pyrimidines]. We discovered that the number of HeLa cells with filopodium-like membrane protrusions was reduced significantly (from 91% in control cells to 35%) after treatment with the most active compounds. A decrease in cell motility was also noticed. Preliminary in vivo experiments on the impact of the studied compounds on the dynamics of CT26 tumor growth in Balb/C mice were also performed.
Keywords: 3-azaspiro[bicyclo[3.1.0]hexane-2,5′-pyrimidines]; CT26; HeLa; Jurkat; Vero); alloxan-derived azomethine ylide; antiproliferative activity; cancer cell lines (K-562; cell cycle; cell death; cell motility; cyclopropenes; in vitro and in vivo activity; morphological changes (cytoskeleton).
Conflict of interest statement
The authors declare no conflict of interest.
Figures



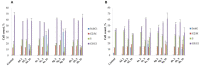



Similar articles
-
Identification of Spiro-Fused Pyrrolo[3,4-a]pyrrolizines and Tryptanthrines as Potential Antitumor Agents: Synthesis and In Vitro Evaluation.Int J Mol Sci. 2021 Nov 5;22(21):11997. doi: 10.3390/ijms222111997. Int J Mol Sci. 2021. PMID: 34769424 Free PMC article.
-
Identification of Spiro-Fused [3-azabicyclo[3.1.0]hexane]oxindoles as Potential Antitumor Agents: Initial In Vitro Evaluation of Anti-Proliferative Effect and Actin Cytoskeleton Transformation in 3T3 and 3T3-SV40 Fibroblast.Int J Mol Sci. 2021 Jul 31;22(15):8264. doi: 10.3390/ijms22158264. Int J Mol Sci. 2021. PMID: 34361029 Free PMC article.
-
Micronucleus-inducing and antitumor activity of three newly synthesized bridged nitrogen atom-containing pyrimidines.J BUON. 2006 Jul-Sep;11(3):329-34. J BUON. 2006. PMID: 17309158
-
Design, Synthesis, biological Evaluation, and molecular docking studies of novel Pyrazolo[3,4-d]Pyrimidine derivative scaffolds as potent EGFR inhibitors and cell apoptosis inducers.Bioorg Chem. 2021 Nov;116:105325. doi: 10.1016/j.bioorg.2021.105325. Epub 2021 Sep 4. Bioorg Chem. 2021. PMID: 34507234
-
Can dicoumarol be used as a gonad-safe anticancer agent: an in vitro and in vivo experimental study.Mol Hum Reprod. 2016 Jan;22(1):57-67. doi: 10.1093/molehr/gav065. Epub 2015 Nov 26. Mol Hum Reprod. 2016. PMID: 26612783
Cited by
-
Design, synthesis, pharmacological evaluation, and in silico studies of the activity of novel spiro pyrrolo[3,4-d]pyrimidine derivatives.RSC Adv. 2024 Jan 2;14(2):995-1008. doi: 10.1039/d3ra07078f. eCollection 2024 Jan 2. RSC Adv. 2024. PMID: 38174254 Free PMC article.
References
-
- Lahlou M. The Success of Natural Products in Drug Discovery. Pharmacol. Pharm. 2013;4:17–31. doi: 10.4236/pp.2013.43A003. - DOI
-
- Srinivasulu V., Schilf P., Ibrahim S., Khanfar M.A., Sieburth S.M., Omar H., Sebastian A., AlQawasmeh R.A., O’Connor M.J., Al-Tel T.H. Multidirectional desymmetrization of pluripotent building block en route to diastereoselective synthesis of complex nature-inspired scaffolds. Nat. Commun. 2018;9:4989. doi: 10.1038/s41467-018-07521-2. - DOI - PMC - PubMed
-
- Tanaka S., Honmura Y., Uesugi S., Fukushi E., Tanaka K., Maeda H., Kimura K., Nehira T., Hashimoto M. Cyclohelminthol X, a Hexa-Substituted Spirocyclopropane from Helminthosporium velutinum yone96: Structural Elucidation, Electronic Circular Dichroism Analysis, and Biological Properties. J. Org. Chem. 2017;82:5574–5582. doi: 10.1021/acs.joc.7b00393. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

