SARS-CoV-2 Spike Proteins and Cell-Cell Communication Inhibits TFPI and Induces Thrombogenic Factors in Human Lung Microvascular Endothelial Cells and Neutrophils: Implications for COVID-19 Coagulopathy Pathogenesis
- PMID: 36142345
- PMCID: PMC9499475
- DOI: 10.3390/ijms231810436
SARS-CoV-2 Spike Proteins and Cell-Cell Communication Inhibits TFPI and Induces Thrombogenic Factors in Human Lung Microvascular Endothelial Cells and Neutrophils: Implications for COVID-19 Coagulopathy Pathogenesis
Abstract
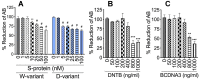
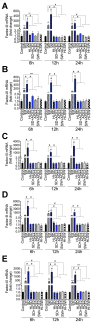
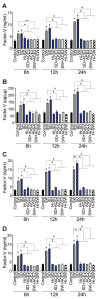
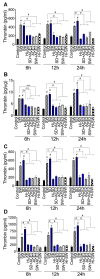
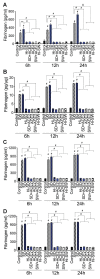
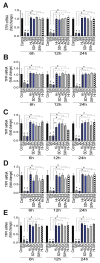
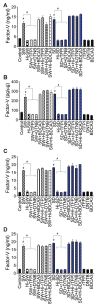
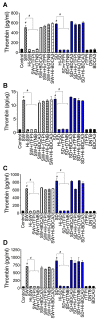
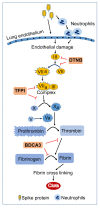
In SARS-CoV-2-infected humans, disease progression is often associated with acute respiratory distress syndrome involving severe lung injury, coagulopathy, and thrombosis of the alveolar capillaries. The pathogenesis of these pulmonary complications in COVID-19 patients has not been elucidated. Autopsy study of these patients showed SARS-CoV-2 virions in pulmonary vessels and sequestrated leukocytes infiltrates associated with endotheliopathy and microvascular thrombosis. Since SARS-CoV-2 enters and infects target cells by binding its spike (S) protein to cellular angiotensin-converting enzyme 2 (ACE2), and there is evidence that vascular endothelial cells and neutrophils express ACE2, we investigated the effect of S-proteins and cell-cell communication on primary human lung microvascular endothelial cells (HLMEC) and neutrophils expression of thrombogenic factors and the potential mechanisms. Using S-proteins of two different SARS-CoV-2 variants (Wuhan and Delta), we demonstrate that exposure of HLMEC or neutrophils to S-proteins, co-culture of HLMEC exposed to S-proteins with non-exposed neutrophils, or co-culture of neutrophils exposed to S-proteins with non-exposed HLMEC induced transcriptional upregulation of tissue factor (TF), significantly increased the expression and secretion of factor (F)-V, thrombin, and fibrinogen and inhibited tissue factor pathway inhibitor (TFPI), the primary regulator of the extrinsic pathway of blood coagulation, in both cell types. Recombinant (r)TFPI and a thiol blocker (5,5'-dithio-bis-(2-nitrobenzoic acid)) prevented S-protein-induced expression and secretion of Factor-V, thrombin, and fibrinogen. Thrombomodulin blocked S-protein-induced expression and secretion of fibrinogen but had no effect on S-protein-induced expression of Factor-V or thrombin. These results suggests that following SARS-CoV-2 contact with the pulmonary endothelium or neutrophils and endothelial-neutrophil interactions, viral S-proteins induce coagulopathy via the TF pathway and mechanisms involving functional thiol groups. These findings suggest that using rTFPI and/or thiol-based drugs could be a viable therapeutic strategy against SARS-CoV-2-induced coagulopathy and thrombosis.
Keywords: DTNB; Factor-V; SARS-CoV-2 spike proteins; TFPI; fibrinogen; human lung endothelial cells; neutrophils; thrombin; thrombomodulin; tissue factor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
SARS-CoV-2 Spike Proteins and Cell-Cell Communication Induce P-Selectin and Markers of Endothelial Injury, NETosis, and Inflammation in Human Lung Microvascular Endothelial Cells and Neutrophils: Implications for the Pathogenesis of COVID-19 Coagulopathy.Int J Mol Sci. 2023 Aug 9;24(16):12585. doi: 10.3390/ijms241612585. Int J Mol Sci. 2023. PMID: 37628764 Free PMC article.
-
SARS-CoV-2 Spike Protein Destabilizes Microvascular Homeostasis.Microbiol Spectr. 2021 Dec 22;9(3):e0073521. doi: 10.1128/Spectrum.00735-21. Epub 2021 Dec 22. Microbiol Spectr. 2021. PMID: 34935423 Free PMC article.
-
SARS-CoV-2 Spike Protein 1 Activates Microvascular Endothelial Cells and Complement System Leading to Platelet Aggregation.Front Immunol. 2022 Mar 7;13:827146. doi: 10.3389/fimmu.2022.827146. eCollection 2022. Front Immunol. 2022. PMID: 35320941 Free PMC article.
-
Endothelium Infection and Dysregulation by SARS-CoV-2: Evidence and Caveats in COVID-19.Viruses. 2020 Dec 26;13(1):29. doi: 10.3390/v13010029. Viruses. 2020. PMID: 33375371 Free PMC article. Review.
-
The coagulopathy, endotheliopathy, and vasculitis of COVID-19.Inflamm Res. 2020 Dec;69(12):1181-1189. doi: 10.1007/s00011-020-01401-6. Epub 2020 Sep 12. Inflamm Res. 2020. PMID: 32918567 Free PMC article. Review.
Cited by
-
Mechanisms of endothelial activation, hypercoagulation and thrombosis in COVID-19: a link with diabetes mellitus.Cardiovasc Diabetol. 2024 Feb 20;23(1):75. doi: 10.1186/s12933-023-02097-8. Cardiovasc Diabetol. 2024. PMID: 38378550 Free PMC article. Review.
-
Relevance of Spike/Estrogen Receptor-α interaction for endothelial-based coagulopathy induced by SARS-CoV-2.Signal Transduct Target Ther. 2023 May 19;8(1):203. doi: 10.1038/s41392-023-01488-3. Signal Transduct Target Ther. 2023. PMID: 37208343 Free PMC article. No abstract available.
-
SARS-CoV-2 Spike Proteins and Cell-Cell Communication Induce P-Selectin and Markers of Endothelial Injury, NETosis, and Inflammation in Human Lung Microvascular Endothelial Cells and Neutrophils: Implications for the Pathogenesis of COVID-19 Coagulopathy.Int J Mol Sci. 2023 Aug 9;24(16):12585. doi: 10.3390/ijms241612585. Int J Mol Sci. 2023. PMID: 37628764 Free PMC article.
-
Extracellular Vesicle-Based SARS-CoV-2 Vaccine.Vaccines (Basel). 2023 Feb 24;11(3):539. doi: 10.3390/vaccines11030539. Vaccines (Basel). 2023. PMID: 36992123 Free PMC article. Review.
References
-
- Peeri N.C., Shrestha N., Rahman M.S., Zaki R., Tan Z., Bibi S., Baghbanzadeh M., Aghamohammadi N., Zhang W., Haque U. The SARS, MERS and novel coronavirus (COVID-19) epidemics, the newest and biggest global health threats: What lessons have we learned? Int. J. Epidemiol. 2020;49:717–726. doi: 10.1093/ije/dyaa033. - DOI - PMC - PubMed
-
- JHU Coronavirus Resource Center: COVID-19 in the USA. 2022. [(accessed on 22 August 2022)]. Available online: https://coronavirus.jhu.edu/
-
- CDC United States COVID-19 Cases and Deaths by State. US Center for Disease Control and Prevention. [(accessed on 22 August 2022)];2022 Available online: https://www.cdc.gov/covid-data-tracker/#cases.
-
- WHO Coronavirus disease (COVID-19) pandemic. World Health Organization. 2022. [(accessed on 22 August 2022)]. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019.
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

