ZBP1: A Powerful Innate Immune Sensor and Double-Edged Sword in Host Immunity
- PMID: 36142136
- PMCID: PMC9499459
- DOI: 10.3390/ijms231810224
ZBP1: A Powerful Innate Immune Sensor and Double-Edged Sword in Host Immunity
Abstract
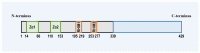
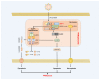
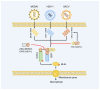
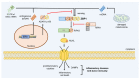
Z-conformation nucleic acid binding protein 1 (ZBP1), a powerful innate immune sensor, has been identified as the important signaling initiation factor in innate immune response and the multiple inflammatory cell death known as PANoptosis. The initiation of ZBP1 signaling requires recognition of left-handed double-helix Z-nucleic acid (includes Z-DNA and Z-RNA) and subsequent signaling transduction depends on the interaction between ZBP1 and its adapter proteins, such as TANK-binding kinase 1 (TBK1), interferon regulatory factor 3 (IRF3), receptor-interacting serine/threonine-protein kinase 1 (RIPK1), and RIPK3. ZBP1 activated innate immunity, including type-I interferon (IFN-I) response and NF-κB signaling, constitutes an important line of defense against pathogenic infection. In addition, ZBP1-mediated PANoptosis is a double-edged sword in anti-infection, auto-inflammatory diseases, and tumor immunity. ZBP1-mediated PANoptosis is beneficial for eliminating infected cells and tumor cells, but abnormal or excessive PANoptosis can lead to a strong inflammatory response that is harmful to the host. Thus, pathogens and host have each developed multiplex tactics targeting ZBP1 signaling to maintain strong virulence or immune homeostasis. In this paper, we reviewed the mechanisms of ZBP1 signaling, the effects of ZBP1 signaling on host immunity and pathogen infection, and various antagonistic strategies of host and pathogen against ZBP1. We also discuss existent gaps regarding ZBP1 signaling and forecast potential directions for future research.
Keywords: ZBP1; apoptosis; auto-inflammatory diseases; innate immunity; necroptosis; pathogen–host interactions; pyroptosis; signaling transduction; tumor immunity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Z-DNA binding protein 1 orchestrates innate immunity and inflammatory cell death.Cytokine Growth Factor Rev. 2024 Jun;77:15-29. doi: 10.1016/j.cytogfr.2024.03.005. Epub 2024 Mar 26. Cytokine Growth Factor Rev. 2024. PMID: 38548490 Review.
-
Recent advances in ZBP1-derived PANoptosis against viral infections.Front Immunol. 2023 May 16;14:1148727. doi: 10.3389/fimmu.2023.1148727. eCollection 2023. Front Immunol. 2023. PMID: 37261341 Free PMC article. Review.
-
The regulation of the ZBP1-NLRP3 inflammasome and its implications in pyroptosis, apoptosis, and necroptosis (PANoptosis).Immunol Rev. 2020 Sep;297(1):26-38. doi: 10.1111/imr.12909. Epub 2020 Jul 29. Immunol Rev. 2020. PMID: 32729116 Free PMC article. Review.
-
Z-nucleic-acid sensing triggers ZBP1-dependent necroptosis and inflammation.Nature. 2020 Apr;580(7803):391-395. doi: 10.1038/s41586-020-2129-8. Epub 2020 Mar 25. Nature. 2020. PMID: 32296175 Free PMC article.
-
The Zα2 domain of ZBP1 is a molecular switch regulating influenza-induced PANoptosis and perinatal lethality during development.J Biol Chem. 2020 Jun 12;295(24):8325-8330. doi: 10.1074/jbc.RA120.013752. Epub 2020 Apr 29. J Biol Chem. 2020. PMID: 32350114 Free PMC article.
Cited by
-
RIPK3 signaling and its role in regulated cell death and diseases.Cell Death Discov. 2024 Apr 29;10(1):200. doi: 10.1038/s41420-024-01957-w. Cell Death Discov. 2024. PMID: 38684668 Free PMC article. Review.
-
PANoptosis: A Cell Death Characterized by Pyroptosis, Apoptosis, and Necroptosis.J Inflamm Res. 2023 Apr 12;16:1523-1532. doi: 10.2147/JIR.S403819. eCollection 2023. J Inflamm Res. 2023. PMID: 37077221 Free PMC article. Review.
-
ZBP1 inhibits the replication of Senecavirus A by enhancing NF-κB signaling pathway mediated antiviral response in porcine alveolar macrophage 3D4/21 cells.Cell Mol Biol Lett. 2024 May 31;29(1):83. doi: 10.1186/s11658-024-00598-2. Cell Mol Biol Lett. 2024. PMID: 38822277 Free PMC article.
-
Single-cell RNA sequencing reveals Immune Education promotes T cell survival in mice subjected to the cecal ligation and puncture sepsis model.Front Immunol. 2024 Mar 18;15:1366955. doi: 10.3389/fimmu.2024.1366955. eCollection 2024. Front Immunol. 2024. PMID: 38562928 Free PMC article.
-
Understanding nucleic acid sensing and its therapeutic applications.Exp Mol Med. 2023 Nov;55(11):2320-2331. doi: 10.1038/s12276-023-01118-6. Epub 2023 Nov 9. Exp Mol Med. 2023. PMID: 37945923 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

