The Discovery and Development of Natural-Based Biomaterials with Demonstrated Wound Healing Properties: A Reliable Approach in Clinical Trials
- PMID: 36140332
- PMCID: PMC9496351
- DOI: 10.3390/biomedicines10092226
The Discovery and Development of Natural-Based Biomaterials with Demonstrated Wound Healing Properties: A Reliable Approach in Clinical Trials
Abstract
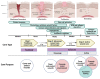
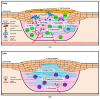
Current research across the globe still focuses strongly on naturally derived biomaterials in various fields, particularly wound care. There is a need for more effective therapies that will address the physiological deficiencies underlying chronic wound treatment. The use of moist bioactive scaffolds has significantly increased healing rates compared to local and traditional treatments. However, failure to heal or prolonging the wound healing process results in increased financial and social stress imposed on health institutions, caregivers, patients, and their families. The urgent need to identify practical, safe, and cost-effective wound healing scaffolding from natural-based biomaterials that can be introduced into clinical practice is unequivocal. Naturally derived products have long been used in wound healing; however, clinical trial evaluations of these therapies are still in their infancy. Additionally, further well-designed clinical trials are necessary to confirm the efficacy and safety of natural-based biomaterials in treating wounds. Thus, the focus of this review is to describe the current insight, the latest discoveries in selected natural-based wound healing implant products, the possible action mechanisms, and an approach to clinical studies. We explore several tested products undergoing clinical trials as a novel approach to counteract the debilitating effects of impaired wound healing.
Keywords: biomaterial; clinical trial; dressings; natural products; tissue-engineered skin; wound healing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Advanced Therapeutic Dressings for Effective Wound Healing--A Review.J Pharm Sci. 2015 Nov;104(11):3653-3680. doi: 10.1002/jps.24610. Epub 2015 Aug 26. J Pharm Sci. 2015. PMID: 26308473 Review.
-
A review of the current state of natural biomaterials in wound healing applications.Front Bioeng Biotechnol. 2024 Mar 27;12:1309541. doi: 10.3389/fbioe.2024.1309541. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 38600945 Free PMC article. Review.
-
Phenotypic Screening Identifies Synergistically Acting Natural Product Enhancing the Performance of Biomaterial Based Wound Healing.Front Pharmacol. 2017 Jul 18;8:433. doi: 10.3389/fphar.2017.00433. eCollection 2017. Front Pharmacol. 2017. PMID: 28769790 Free PMC article.
-
Healing of Chronic Wounds: An Update of Recent Developments and Future Possibilities.Tissue Eng Part B Rev. 2019 Oct;25(5):429-444. doi: 10.1089/ten.TEB.2019.0019. Epub 2019 Sep 11. Tissue Eng Part B Rev. 2019. PMID: 31068101 Review.
-
Trends in the Treatment of Chronic Wounds.Curr Med Chem. 2024 Sep 13. doi: 10.2174/0109298673312649240829103906. Online ahead of print. Curr Med Chem. 2024. PMID: 39279699
Cited by
-
Functionalised-biomatrix for wound healing and cutaneous regeneration: future impactful medical products in clinical translation and precision medicine.Front Bioeng Biotechnol. 2023 May 24;11:1160577. doi: 10.3389/fbioe.2023.1160577. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 37292094 Free PMC article. Review.
-
Why Are There So Few FDA-Approved Therapeutics for Wound Healing?Int J Mol Sci. 2023 Oct 12;24(20):15109. doi: 10.3390/ijms242015109. Int J Mol Sci. 2023. PMID: 37894789 Free PMC article. Review.
-
Antioxidant Biomaterials in Cutaneous Wound Healing and Tissue Regeneration: A Critical Review.Antioxidants (Basel). 2023 Mar 23;12(4):787. doi: 10.3390/antiox12040787. Antioxidants (Basel). 2023. PMID: 37107164 Free PMC article. Review.
-
Effect of Multiple-Cycle Collections of Conditioned Media from Different Cell Sources towards Fibroblasts in In Vitro Wound Healing Model.Pharmaceutics. 2024 Jun 5;16(6):767. doi: 10.3390/pharmaceutics16060767. Pharmaceutics. 2024. PMID: 38931888 Free PMC article.
-
Natural Compounds and Biomimetic Engineering to Influence Fibroblast Behavior in Wound Healing.Int J Mol Sci. 2024 Mar 14;25(6):3274. doi: 10.3390/ijms25063274. Int J Mol Sci. 2024. PMID: 38542247 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

