Leveraging the Dynamic Immune Environment Triad in Patients with Breast Cancer: Tumour, Lymph Node, and Peripheral Blood
- PMID: 36139665
- PMCID: PMC9496983
- DOI: 10.3390/cancers14184505
Leveraging the Dynamic Immune Environment Triad in Patients with Breast Cancer: Tumour, Lymph Node, and Peripheral Blood
Abstract
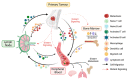
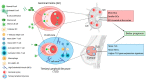
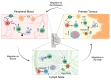
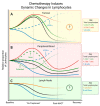
During the anti-tumour response to breast cancer, the primary tumour, the peripheral blood, and the lymph nodes each play unique roles. Immunological features at each site reveal evidence of continuous immune cross-talk between them before, during and after treatment. As such, immune responses to breast cancer are found to be highly dynamic and truly systemic, integrating three distinct immune sites, complex cell-migration highways, as well as the temporal dimension of disease progression and treatment. In this review, we provide a connective summary of the dynamic immune environment triad of breast cancer. It is critical that future studies seek to establish dynamic immune profiles, constituting multiple sites, that capture the systemic immune response to breast cancer and define patient-selection parameters resulting in more significant overall responses and survival rates for breast cancer patients.
Keywords: breast cancer; lymph node; systemic immunity; triple-negative breast cancers.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures
Similar articles
-
Genetic and immune characteristics of sentinel lymph node metastases and multiple lymph node metastases compared to their matched primary breast tumours.EBioMedicine. 2021 Sep;71:103542. doi: 10.1016/j.ebiom.2021.103542. Epub 2021 Aug 26. EBioMedicine. 2021. PMID: 34454403 Free PMC article.
-
Histological scoring of immune and stromal features in breast and axillary lymph nodes is prognostic for distant metastasis in lymph node-positive breast cancers.J Pathol Clin Res. 2018 Jan 8;4(1):39-54. doi: 10.1002/cjp2.87. eCollection 2018 Jan. J Pathol Clin Res. 2018. PMID: 29416876 Free PMC article.
-
Overview of resistance to systemic therapy in patients with breast cancer.Adv Exp Med Biol. 2007;608:1-22. doi: 10.1007/978-0-387-74039-3_1. Adv Exp Med Biol. 2007. PMID: 17993229 Review.
-
Decreased expression of key tumour suppressor microRNAs is associated with lymph node metastases in triple negative breast cancer.BMC Cancer. 2014 Jan 31;14:51. doi: 10.1186/1471-2407-14-51. BMC Cancer. 2014. PMID: 24479446 Free PMC article.
-
Molecular patterns of cancer colonisation in lymph nodes of breast cancer patients.Breast Cancer Res. 2018 Nov 20;20(1):143. doi: 10.1186/s13058-018-1070-3. Breast Cancer Res. 2018. PMID: 30458865 Free PMC article. Review.
Cited by
-
Detailed Profiling of the Tumor Microenvironment in Ethnic Breast Cancer, Using Tissue Microarrays and Multiplex Immunofluorescence.Int J Mol Sci. 2024 Jun 13;25(12):6501. doi: 10.3390/ijms25126501. Int J Mol Sci. 2024. PMID: 38928207 Free PMC article.
-
Baseline splenic volume as a biomarker for clinical outcome and circulating lymphocyte count in gastric cancer.Front Oncol. 2023 Jan 30;12:1065716. doi: 10.3389/fonc.2022.1065716. eCollection 2022. Front Oncol. 2023. PMID: 36793344 Free PMC article.
-
Immune checkpoint status and exhaustion-related phenotypes of CD8+ T cells from the tumor-draining regional lymph nodes in breast cancer.Cancer Med. 2023 Dec;12(24):22196-22205. doi: 10.1002/cam4.6802. Epub 2023 Dec 8. Cancer Med. 2023. PMID: 38069525 Free PMC article.
References
-
- Schmid P., Adams S., Rugo H.S., Schneeweiss A., Barrios C.H., Iwata H., Dieras V., Henschel V., Molinero L., Chui S.Y., et al. IMpassion130: Updated overall survival (OS) from a global, randomized, double-blind, placebo-controlled, Phase III study of atezolizumab (atezo) + nab-paclitaxel (nP) in previously untreated locally advanced or metastatic triple-negative breast cancer (mTNBC) J. Clin. Oncol. 2019;37:1003. doi: 10.1200/jco.2019.37.15_suppl.1003. - DOI
-
- Cortes J., Cescon D.W., Rugo H.S., Nowecki Z., Im S.-A., Yusof M., Gallardo C., Lipatov O., Barrios C.H., Holgado E., et al. KEYNOTE-355: Randomized, double-blind, phase III study of pembrolizumab + chemotherapy versus placebo + chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer. J. Clin. Oncol. 2020;38:1000. doi: 10.1200/JCO.2020.38.15_suppl.1000. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

