Regulating the Expression of HIF-1α or lncRNA: Potential Directions for Cancer Therapy
- PMID: 36139386
- PMCID: PMC9496732
- DOI: 10.3390/cells11182811
Regulating the Expression of HIF-1α or lncRNA: Potential Directions for Cancer Therapy
Abstract
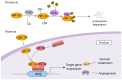
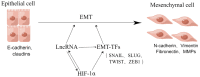
Previous studies have shown that tumors under a hypoxic environment can induce an important hypoxia-responsive element, hypoxia-induced factor-1α (HIF-1α), which can increase tumor migration, invasion, and metastatic ability by promoting epithelial-to-mesenchymal transition (EMT) in tumor cells. Currently, with the deeper knowledge of long noncoding RNAs (lncRNAs), more and more functions of lncRNAs have been discovered. HIF-1α can regulate hypoxia-responsive lncRNAs under hypoxic conditions, and changes in the expression level of lncRNAs can regulate the production of EMT transcription factors and signaling pathway transduction, thus promoting EMT progress. In conclusion, this review summarizes the regulation of the EMT process by HIF-1α and lncRNAs and discusses their relationship with tumorigenesis. Since HIF-1α plays an important role in tumor progression, we also summarize the current drugs that inhibit tumor progression by modulating HIF-1α.
Keywords: EMT; HIF-1α; HIF-1α inhibitors; hypoxia; lncRNAs.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Long non-coding RNA HOTTIP promotes hypoxia-induced epithelial-mesenchymal transition of malignant glioma by regulating the miR-101/ZEB1 axis.Biomed Pharmacother. 2017 Nov;95:711-720. doi: 10.1016/j.biopha.2017.08.133. Epub 2017 Sep 5. Biomed Pharmacother. 2017. PMID: 28886531
-
Hypoxia-induced lncRNA RP11-390F4.3 promotes epithelial-mesenchymal transition (EMT) and metastasis through upregulating EMT regulators.Cancer Lett. 2020 Jul 28;483:35-45. doi: 10.1016/j.canlet.2020.04.014. Epub 2020 Apr 27. Cancer Lett. 2020. PMID: 32353468
-
Upregulation of the long noncoding RNA UBOX5 antisense RNA 1 (UBOX5-AS1) under hypoxic conditions promotes epithelial-mesenchymal transition in endometriosis.Ann Transl Med. 2021 May;9(9):790. doi: 10.21037/atm-20-4546. Ann Transl Med. 2021. PMID: 34268403 Free PMC article.
-
Interplay of long non-coding RNAs and HIF-1α: A new dimension to understanding hypoxia-regulated tumor growth and metastasis.Cancer Lett. 2021 Feb 28;499:49-59. doi: 10.1016/j.canlet.2020.11.007. Epub 2020 Nov 18. Cancer Lett. 2021. PMID: 33217445 Review.
-
The role of noncoding RNAs in the tumor microenvironment of hepatocellular carcinoma.Acta Biochim Biophys Sin (Shanghai). 2023 Nov 25;55(11):1697-1706. doi: 10.3724/abbs.2023231. Acta Biochim Biophys Sin (Shanghai). 2023. PMID: 37867435 Free PMC article. Review.
Cited by
-
Maternal NO2 exposure and fetal growth restriction: Hypoxia transmission and lncRNAs-proinflammation-mediated abnormal hematopoiesis.Proc Natl Acad Sci U S A. 2024 Oct 29;121(44):e2409597121. doi: 10.1073/pnas.2409597121. Epub 2024 Oct 21. Proc Natl Acad Sci U S A. 2024. PMID: 39432779
-
Hypoxia induced cellular and exosomal RPPH1 promotes breast cancer angiogenesis and metastasis through stabilizing the IGF2BP2/FGFR2 axis.Oncogene. 2025 Feb;44(3):147-164. doi: 10.1038/s41388-024-03213-y. Epub 2024 Nov 4. Oncogene. 2025. PMID: 39496940
-
Hypoxia-Inducible Factor 1-Alpha (HIF-1α): An Essential Regulator in Cellular Metabolic Control.Cureus. 2024 Jul 4;16(7):e63852. doi: 10.7759/cureus.63852. eCollection 2024 Jul. Cureus. 2024. PMID: 39099978 Free PMC article. Review.
-
Molecular mechanism of oroxyli semen against triple-negative breast cancer verified by bioinformatics and in vitro experiments.Medicine (Baltimore). 2023 Sep 15;102(37):e34835. doi: 10.1097/MD.0000000000034835. Medicine (Baltimore). 2023. PMID: 37713894 Free PMC article.
-
Targeting ARNT attenuates chemoresistance through destabilizing p38α-MAPK signaling in glioblastoma.Cell Death Dis. 2024 May 28;15(5):366. doi: 10.1038/s41419-024-06735-1. Cell Death Dis. 2024. PMID: 38806469 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

