Peptidomimetics and Their Applications for Opioid Peptide Drug Discovery
- PMID: 36139079
- PMCID: PMC9496382
- DOI: 10.3390/biom12091241
Peptidomimetics and Their Applications for Opioid Peptide Drug Discovery
Abstract
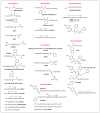
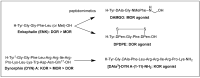
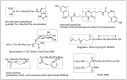
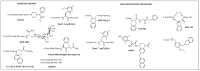
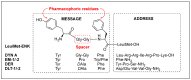
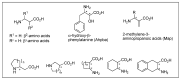
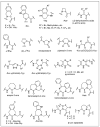
Despite various advantages, opioid peptides have been limited in their therapeutic uses due to the main drawbacks in metabolic stability, blood-brain barrier permeability, and bioavailability. Therefore, extensive studies have focused on overcoming the problems and optimizing the therapeutic potential. Currently, numerous peptide-based drugs are being marketed thanks to new synthetic strategies for optimizing metabolism and alternative routes of administration. This tutorial review briefly introduces the history and role of natural opioid peptides and highlights the key findings on their structure-activity relationships for the opioid receptors. It discusses details on opioid peptidomimetics applied to develop therapeutic candidates for the treatment of pain from the pharmacological and structural points of view. The main focus is the current status of various mimetic tools and the successful applications summarized in tables and figures.
Keywords: analgesic drugs; bioavailability; locally constrained peptides; opioid receptors; peptide backbone modifications; peptidomimetic.
Conflict of interest statement
The author declares no conflict of interest.
Figures
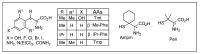
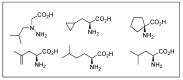
Similar articles
-
Multivalent peptide and peptidomimetic ligands for the treatment of pain without toxicities and addiction.Peptides. 2019 Jun;116:63-67. doi: 10.1016/j.peptides.2019.02.004. Epub 2019 Apr 20. Peptides. 2019. PMID: 31014958 Review.
-
Development of opioid peptide analogs for pain relief.Curr Pharm Des. 2010;16(9):1126-35. doi: 10.2174/138161210790963869. Curr Pharm Des. 2010. PMID: 20030621 Review.
-
Discovery of two novel branched peptidomimetics containing endomorphin-2 and RF9 pharmacophores: Synthesis and neuropharmacological evaluation.Bioorg Med Chem. 2019 Feb 15;27(4):630-643. doi: 10.1016/j.bmc.2019.01.003. Epub 2019 Jan 4. Bioorg Med Chem. 2019. PMID: 30626554
-
New trends in the development of opioid peptide analogues as advanced remedies for pain relief.Curr Top Med Chem. 2004;4(1):19-38. doi: 10.2174/1568026043451663. Curr Top Med Chem. 2004. PMID: 14754374 Review.
-
Pain: nocistatin spells relief.Curr Biol. 1998 Jul 16;8(15):R525-7. doi: 10.1016/s0960-9822(07)00338-7. Curr Biol. 1998. PMID: 9705926 Review.
Cited by
-
Analgesic Peptides: From Natural Diversity to Rational Design.Molecules. 2024 Mar 29;29(7):1544. doi: 10.3390/molecules29071544. Molecules. 2024. PMID: 38611824 Free PMC article. Review.
-
Therapeutic Potential of Orally Administered Rubiscolin-6.Int J Mol Sci. 2023 Jun 9;24(12):9959. doi: 10.3390/ijms24129959. Int J Mol Sci. 2023. PMID: 37373107 Free PMC article. Review.
-
In Vitro and In Vivo Pharmacological Profiles of LENART01, a Dermorphin-Ranatensin Hybrid Peptide.Int J Mol Sci. 2024 Apr 3;25(7):4007. doi: 10.3390/ijms25074007. Int J Mol Sci. 2024. PMID: 38612817 Free PMC article.
-
Peptide-derived ligands for the discovery of safer opioid analgesics.Drug Discov Today. 2024 May;29(5):103950. doi: 10.1016/j.drudis.2024.103950. Epub 2024 Mar 20. Drug Discov Today. 2024. PMID: 38514040 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

