Attributes in stated preference elicitation studies on colorectal cancer screening and their relative importance for decision-making among screenees: a systematic review
- PMID: 36136248
- PMCID: PMC9494881
- DOI: 10.1186/s13561-022-00394-8
Attributes in stated preference elicitation studies on colorectal cancer screening and their relative importance for decision-making among screenees: a systematic review
Abstract
Introduction: The SIGMO study (Sigmoidoscopy as an evidence-based colorectal cancer screening test - a possible option?) examines screening eligible populations' preferences for colorectal cancer (CRC) screening in Germany using a discrete choice experiment (DCE). Attribute identification and selection are essential for the construction of choice tasks and should be evidence-based. As a part of the SIGMO study this systematic review provides an overview of attributes included in studies eliciting stated preferences for CRC screening tests and their relative importance for decision-making.
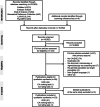
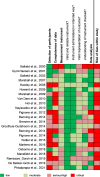
Methods: Systematic search (November 2021) for English-language studies published since January 2000 in PubMed, Embase, Web of Science, Biomedical Reference Collection: Corporate Edition, LIVIVO and PsycINFO. DCEs and conjoint analysis ranking or rating tasks on screening eligible populations' preferences for stool testing, sigmoidoscopy, and/or colonoscopy were included. Attributes were extracted and their relative importance was calculated and ranked. Risk of bias (RoB) of included studies was assessed using a modified GRADE (Grading of Recommendations Assessment, Development and Evaluation) approach. Study selection and RoB rating were carried out independently by two reviewers. Data were extracted by one reviewer and checked by another one.
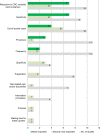
Results: A total of 23 publications on 22 studies were included. Overall RoB was rated as serious/critical for 21 studies and as moderate for 2 studies. Main reasons for high RoB were non-random sampling, low response rates, lack of non-responder analyses, and, to a lesser extent, weaknesses in the measurement instrument and data analysis. Extracted attributes (n = 120) referred to procedure-related characteristics (n = 42; 35%), structural characteristics of health care (n = 24; 20%), test characteristics (n = 23; 19%), harms (n = 16; 13%), benefits (n = 13; 11%), and level of evidence (n = 2; 2%). Most important attributes were reduction in CRC mortality (and incidence) (n = 7), test sensitivity (n = 7), out-of-pocket costs (n = 4), procedure (n = 3), and frequency (n = 2).
Conclusions: Health preference studies on CRC were found to have a high RoB. The composition of choice tasks revealed a lack of attributes on patient-important outcomes (like incidence reduction), while attributes not considered relevant for individual screening decisions (like sensitivity) were frequently used. Future studies eliciting stated preferences in cancer screening should apply the principles of informed decision-making in attribute identification and selection.
Keywords: Colorectal cancer screening; Discrete choice experiment; GRADE; Informed decision-making; Risk of bias; Systematic review.
© 2022. The Author(s).
Conflict of interest statement
MB, LMF, LD, CK, and MD have received research grants from the German Federal Joint Committee’s Innovation Fund. BPR declares that he has no competing interests.
Figures
Similar articles
-
Attributes Characterizing Colorectal Cancer Screening Tests That Influence Preferences of Individuals Eligible for Screening in Germany: A Qualitative Study.Patient Prefer Adherence. 2022 Aug 10;16:2051-2066. doi: 10.2147/PPA.S365429. eCollection 2022. Patient Prefer Adherence. 2022. PMID: 35975173 Free PMC article.
-
A Systematic Review of Discrete Choice Experiments and Conjoint Analysis on Genetic Testing.Patient. 2022 Jan;15(1):39-54. doi: 10.1007/s40271-021-00531-1. Epub 2021 Jun 4. Patient. 2022. PMID: 34085205
-
Older adults' preferences for colorectal cancer-screening test attributes and test choice.Patient Prefer Adherence. 2015 Jul 15;9:1005-16. doi: 10.2147/PPA.S82203. eCollection 2015. Patient Prefer Adherence. 2015. PMID: 26203233 Free PMC article.
-
Screening for Colorectal Cancer: A Systematic Review for the U.S. Preventive Services Task Force [Internet].Rockville (MD): Agency for Healthcare Research and Quality (US); 2016 Jun. Report No.: 14-05203-EF-1. Rockville (MD): Agency for Healthcare Research and Quality (US); 2016 Jun. Report No.: 14-05203-EF-1. PMID: 27441328 Free Books & Documents. Review.
-
Screening for Colorectal Cancer: An Evidence Update for the U.S. Preventive Services Task Force [Internet].Rockville (MD): Agency for Healthcare Research and Quality (US); 2021 May. Report No.: 20-05271-EF-1. Rockville (MD): Agency for Healthcare Research and Quality (US); 2021 May. Report No.: 20-05271-EF-1. PMID: 34097369 Free Books & Documents. Review.
Cited by
-
Comparative effectiveness of two interventions to increase colorectal cancer screening among females living in the rural Midwest.J Rural Health. 2024 Sep;40(4):610-622. doi: 10.1111/jrh.12828. Epub 2024 Feb 23. J Rural Health. 2024. PMID: 38391093 Clinical Trial.
-
Advancing Colorectal Cancer Detection With Blood-Based Tests: Qualitative Study and Discrete Choice Experiment to Elicit Population Preferences.JMIR Public Health Surveill. 2024 Jul 17;10:e53200. doi: 10.2196/53200. JMIR Public Health Surveill. 2024. PMID: 39018093 Free PMC article.
References
-
- Mühlbacher A, Bethge S, Tockhorn A. Measuring preferences in healthcare: introduction to discrete-choice experiments. Gesundh ökon Qual manag. 2013;18(04):159–72. doi: 10.1055/s-0032-1330500. - DOI
-
- U.S. Food and Drug Administration. Patient Preference Information – Voluntary Submission, Review in Premarket Approval Applications, Humanitarian Device Exemption Applications, and De Novo Requests, and Inclusion in Decision Summaries and Device Labeling. Guidance for Industry, Food and Drug Administration Staff, and Other Stakeholders. 2016. https://www.fda.gov/media/92593/download. Accessed 5 Feb 2021.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

