Covalent organic functionalization of graphene nanosheets and reduced graphene oxide via 1,3-dipolar cycloaddition of azomethine ylide
- PMID: 36132665
- PMCID: PMC9418116
- DOI: 10.1039/d1na00335f
Covalent organic functionalization of graphene nanosheets and reduced graphene oxide via 1,3-dipolar cycloaddition of azomethine ylide
Erratum in
-
Correction: Covalent organic functionalization of graphene nanosheets and reduced graphene oxide via 1,3-dipolar cycloaddition of azomethine ylide.Nanoscale Adv. 2021 Oct 6;3(21):6242. doi: 10.1039/d1na90092g. eCollection 2021 Oct 27. Nanoscale Adv. 2021. PMID: 36136413 Free PMC article.
Abstract
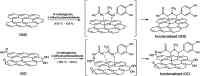
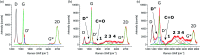
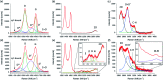
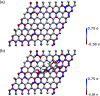
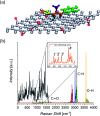
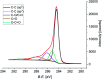
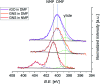
Organic functionalization of graphene is successfully performed via 1,3-dipolar cycloaddition of azomethine ylide in the liquid phase. The comparison between 1-methyl-2-pyrrolidinone and N,N-dimethylformamide as dispersant solvents, and between sonication and homogenization as dispersion techniques, proves N,N-dimethylformamide and homogenization as the most effective choice. The functionalization of graphene nanosheets and reduced graphene oxide is confirmed using different techniques. Among them, energy-dispersive X-ray spectroscopy allows to map the pyrrolidine ring of the azomethine ylide on the surface of functionalized graphene, while micro-Raman spectroscopy detects new features arising from the functionalization, which are described in agreement with the power spectrum obtained from ab initio molecular dynamics simulation. Moreover, X-ray photoemission spectroscopy of functionalized graphene allows the quantitative elemental analysis and the estimation of the surface coverage, showing a higher degree of functionalization for reduced graphene oxide. This more reactive behavior originates from the localization of partial charges on its surface due to the presence of oxygen defects, as shown by the simulation of the electrostatic features. Functionalization of graphene using 1,3-dipolar cycloaddition is shown to be a significant step towards the controlled synthesis of graphene-based complex structures and devices at the nanoscale.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

Similar articles
-
Correction: Covalent organic functionalization of graphene nanosheets and reduced graphene oxide via 1,3-dipolar cycloaddition of azomethine ylide.Nanoscale Adv. 2021 Oct 6;3(21):6242. doi: 10.1039/d1na90092g. eCollection 2021 Oct 27. Nanoscale Adv. 2021. PMID: 36136413 Free PMC article.
-
Shedding Light on the Chemistry and the Properties of Münchnone Functionalized Graphene.Nanomaterials (Basel). 2021 Jun 22;11(7):1629. doi: 10.3390/nano11071629. Nanomaterials (Basel). 2021. PMID: 34206184 Free PMC article.
-
Functionalization of graphene via 1,3-dipolar cycloaddition.ACS Nano. 2010 Jun 22;4(6):3527-33. doi: 10.1021/nn100883p. ACS Nano. 2010. PMID: 20503982
-
The chemical functionalization of graphene nanoplatelets through solvent-free reaction.RSC Adv. 2018 Sep 28;8(58):33564-33573. doi: 10.1039/c8ra04817g. eCollection 2018 Sep 24. RSC Adv. 2018. PMID: 35548120 Free PMC article.
-
Colloidal 2D nanosheets of MoS2 and other transition metal dichalcogenides through liquid-phase exfoliation.Adv Colloid Interface Sci. 2017 Jul;245:40-61. doi: 10.1016/j.cis.2017.04.014. Epub 2017 Apr 25. Adv Colloid Interface Sci. 2017. PMID: 28477866 Review.
Cited by
-
Preparation of a ruthenium complex covalently bonded to multilayer graphene and its evaluation as a photocatalyst.Nanoscale Adv. 2024 Feb 9;6(6):1648-1652. doi: 10.1039/d4na00075g. eCollection 2024 Mar 12. Nanoscale Adv. 2024. PMID: 38482040 Free PMC article.
-
Carbon Nanomaterial Fluorescent Probes and Their Biological Applications.Chem Rev. 2024 Mar 27;124(6):3085-3185. doi: 10.1021/acs.chemrev.3c00581. Epub 2024 Mar 13. Chem Rev. 2024. PMID: 38478064 Free PMC article. Review.
References
-
- Chang H. Wu H. Adv. Funct. Mater. 2013;23:1984–1997. doi: 10.1002/adfm.201202460. - DOI
LinkOut - more resources
Full Text Sources

