Intratumoral CD73: An immune checkpoint shaping an inhibitory tumor microenvironment and implicating poor prognosis in Chinese melanoma cohorts
- PMID: 36131912
- PMCID: PMC9483101
- DOI: 10.3389/fimmu.2022.954039
Intratumoral CD73: An immune checkpoint shaping an inhibitory tumor microenvironment and implicating poor prognosis in Chinese melanoma cohorts
Abstract
Background: As a novel immune checkpoint, CD73 has been reported to play prominent roles in several malignancies. However, the significance of CD73 in melanoma remains ambiguous. This study sought to reveal the impact of CD73 on the tumor microenvironment (TME) and patients' prognosis, and to investigate whether CD73 could be a therapeutic target in Chinese melanomas, which were dominated by acral and mucosal subtypes.
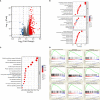
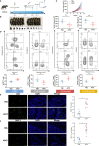
Methods: Two independent Chinese cohorts of 194 patients with melanoma were enrolled. CD73 and PD-L1 expression as well as CD8+ and CD56+ cell infiltrations were evaluated by immunohistochemistry in 194 resected melanoma samples. Clinical outcomes of patients were assessed utilizing the Kaplan-Meier plotter and Cox proportional hazard analysis. RNA-seq data was obtained from TCGA database. Gene set functional annotations were performed based on GO, KEGG and GSEA analysis. CIBERSORT, ssGSEA and TIMER were used to explore the association between CD73 and immune infiltration. These findings were validated by establishing tumor xenograft model, and functions of tumor-infiltrating immune cells were examined by flow cytometry and immunofluorescence.
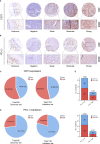
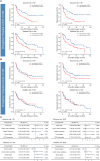
Results: High CD73 expression showed poorer clinical outcomes and was identified as an independent prognostic indicator for survival in two cohorts. Expression of CD73 was more prevalent than PD-L1 in Chinese melanoma cohorts (54.6% vs 23.2%). Co-expression of both immune checkpoints was infrequent (12.9%) in melanoma, and 54.4% of PD-L1 negative cases showed elevated expression of CD73. CD73high tumors showed a microenvironment with fewer CD8+ T cells and CD56+ NK cells infiltration, which displayed a dysfunctional phenotype. With the treatment of CD73 inhibitor APCP, the amount of CD8+ T cells and CD56+ NK cells infiltrated in tumors was elevated and the immunosuppressive effect of CD73 was eliminated.
Conclusions: High CD73 expression was associated with an inhibitory TME and adverse clinical outcomes of melanoma. In comparison to PD-L1, CD73 was more prevalent and possessed more definite prognostic significance. Therefore, it may serve as a prognostic indicator and immunotherapeutic target next to PD-L1 in melanoma for Chinese population.
Keywords: CD73; CD8+ T cells; PD-L1; immunosuppressive; immunotherapy; melanoma; prognosis; tumor microenvironment.
Copyright © 2022 Gao, Wang, Song, Ren, Yang, Li, Shen, Li, Ding, Yang, Zhou, Wei and Gu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
CD73 acts as a prognostic biomarker and promotes progression and immune escape in pancreatic cancer.J Cell Mol Med. 2020 Aug;24(15):8674-8686. doi: 10.1111/jcmm.15500. Epub 2020 Jul 9. J Cell Mol Med. 2020. PMID: 32643277 Free PMC article.
-
Impact of intratumoural CD73 expression on prognosis and therapeutic response in patients with gastric cancer.Eur J Cancer. 2021 Nov;157:114-123. doi: 10.1016/j.ejca.2021.08.006. Epub 2021 Sep 8. Eur J Cancer. 2021. PMID: 34508993
-
Single-Cell Phenotyping of CD73 Expression Reveals the Diversity of the Tumor Immune Microenvironment and Reflects the Prognosis of Bladder Cancer.Lab Invest. 2023 Apr;103(4):100040. doi: 10.1016/j.labinv.2022.100040. Epub 2023 Jan 10. Lab Invest. 2023. PMID: 36870289
-
Prognostic and clinicopathological significance of PD-1/PD-L1 expression in the tumor microenvironment and neoplastic cells for lymphoma.Int Immunopharmacol. 2019 Dec;77:105999. doi: 10.1016/j.intimp.2019.105999. Epub 2019 Nov 6. Int Immunopharmacol. 2019. PMID: 31704289 Review.
-
Inhibitors of the CD73-adenosinergic checkpoint as promising combinatory agents for conventional and advanced cancer immunotherapy.Front Immunol. 2023 Jun 26;14:1212209. doi: 10.3389/fimmu.2023.1212209. eCollection 2023. Front Immunol. 2023. PMID: 37435071 Free PMC article. Review.
Cited by
-
The Roles of MiRNAs (MicroRNAs) in Melanoma Immunotherapy.Int J Mol Sci. 2022 Nov 25;23(23):14775. doi: 10.3390/ijms232314775. Int J Mol Sci. 2022. PMID: 36499102 Free PMC article. Review.
-
Unraveling the Intricacies of CD73/Adenosine Signaling: The Pulmonary Immune and Stromal Microenvironment in Lung Cancer.Cancers (Basel). 2023 Dec 4;15(23):5706. doi: 10.3390/cancers15235706. Cancers (Basel). 2023. PMID: 38067409 Free PMC article. Review.
-
Decoding the metastatic potential and optimal postoperative adjuvant therapy of melanoma based on metastasis score.Cell Death Discov. 2023 Oct 25;9(1):397. doi: 10.1038/s41420-023-01678-6. Cell Death Discov. 2023. PMID: 37880239 Free PMC article.
-
Prediction of survival and immunotherapy response by the combined classifier of G protein-coupled receptors and tumor microenvironment in melanoma.Eur J Med Res. 2023 Sep 16;28(1):352. doi: 10.1186/s40001-023-01346-6. Eur J Med Res. 2023. PMID: 37716991 Free PMC article.
-
Explore the impact of hypoxia-related genes (HRGs) in Cutaneous melanoma.BMC Med Genomics. 2023 Jul 8;16(1):160. doi: 10.1186/s12920-023-01587-8. BMC Med Genomics. 2023. PMID: 37422626 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

