CCL24/CCR3 axis plays a central role in angiotensin II-induced heart failure by stimulating M2 macrophage polarization and fibroblast activation
- PMID: 36131165
- PMCID: PMC10425496
- DOI: 10.1007/s10565-022-09767-5
CCL24/CCR3 axis plays a central role in angiotensin II-induced heart failure by stimulating M2 macrophage polarization and fibroblast activation
Abstract
Aims: We aimed to investigate the effect and mechanism of pleiotropic chemokine CCL24 in heart failure.
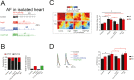
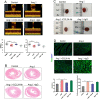
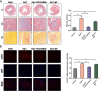
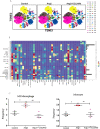
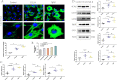
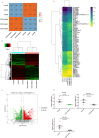
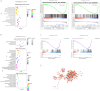
Methods and results: Compared with normal donators, the expression of CCL24 and number of cardiac M2 macrophages in heart were higher in heart failure patients, the same as plasma CCL24. Treatment with CCL24 antibody hindered Ang II (1500 ng/kg/min)-induced cardiac adverse remodeling through preventing cardiac hypertrophy and fibrosis. RNA-seq showed that CCL24/CCR3 axis was involved in immune and inflammatory responses. Single-cell analysis of cytometry by time of flight (CyTOF) revealed that CCL24 antibody decreased the M2 macrophage and monocyte polarization during Ang II stimulation. Immunofluorescence co-localization analysis confirmed the expression of CCR3 in macrophage and fibroblasts. Then, in vitro experiments confirmed that CCL24/CCR3 axis was also involved in cardiac primary fibroblast activation through its G protein-coupled receptor function.
Conclusion: CCL24/CCR3 axis plays a crucial part in cardiac remodeling by stimulating M2 macrophage polarization and cardiac fibroblast activation. Cardiac M2 macrophages, CCL24 and circulation CCL24 increased in heart failure patients. Treatment with CCL24 Ab hindered Ang II induced cardiac structural dysfunction and electrical remodeling. In CCL24 Ab group RNA-seq found that it was related to immune responses and hypertrophic cardiomyopathy, CytoF revealed M2 macrophages and monocytes decreased obviously. In vitro,CCL24 promoted activation and migration of cardiac fibroblast.
Keywords: CCL24; Cardiac fibroblast; Heart failure; M2 macrophage.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
The Role of CCL24 in Systemic Sclerosis.Rambam Maimonides Med J. 2023 Jul 31;14(3):e0016. doi: 10.5041/RMMJ.10504. Rambam Maimonides Med J. 2023. PMID: 37555717 Free PMC article. Review.
-
Trophoblasts-derived chemokine CCL24 promotes the proliferation, growth and apoptosis of decidual stromal cells in human early pregnancy.Int J Clin Exp Pathol. 2013 May 15;6(6):1028-37. Print 2013. Int J Clin Exp Pathol. 2013. PMID: 23696919 Free PMC article.
-
Blocking the autocrine regulatory loop of Gankyrin/STAT3/CCL24/CCR3 impairs the progression and pazopanib resistance of clear cell renal cell carcinoma.Cell Death Dis. 2020 Feb 12;11(2):117. doi: 10.1038/s41419-020-2306-6. Cell Death Dis. 2020. PMID: 32051393 Free PMC article.
-
CXCL1-CXCR2 axis mediates angiotensin II-induced cardiac hypertrophy and remodelling through regulation of monocyte infiltration.Eur Heart J. 2018 May 21;39(20):1818-1831. doi: 10.1093/eurheartj/ehy085. Eur Heart J. 2018. PMID: 29514257
-
The renin-angiotensin system and experimental heart failure.Cardiovasc Res. 1999 Sep;43(4):838-49. doi: 10.1016/s0008-6363(99)00145-5. Cardiovasc Res. 1999. PMID: 10615411 Review.
Cited by
-
Sulfenylation of ERK1/2: A novel mechanism for SO2-mediated inhibition of cardiac fibroblast proliferation.Heliyon. 2024 Jul 6;10(14):e34260. doi: 10.1016/j.heliyon.2024.e34260. eCollection 2024 Jul 30. Heliyon. 2024. PMID: 39092251 Free PMC article.
-
The Role of CCL24 in Systemic Sclerosis.Rambam Maimonides Med J. 2023 Jul 31;14(3):e0016. doi: 10.5041/RMMJ.10504. Rambam Maimonides Med J. 2023. PMID: 37555717 Free PMC article. Review.
-
Multiomic analyses uncover immunological signatures in acute and chronic coronary syndromes.Nat Med. 2024 Jun;30(6):1696-1710. doi: 10.1038/s41591-024-02953-4. Epub 2024 May 21. Nat Med. 2024. PMID: 38773340 Free PMC article.
-
MiR-21-3p inhibitor exerts myocardial protective effects by altering macrophage polarization state and reducing excessive mitophagy.Commun Biol. 2024 Oct 22;7(1):1371. doi: 10.1038/s42003-024-07050-3. Commun Biol. 2024. PMID: 39438580 Free PMC article.
-
Osteoprotegerin Is Essential for the Development of Endothelial Dysfunction Induced by Angiotensin II in Mice.Int J Mol Sci. 2024 Jun 11;25(12):6434. doi: 10.3390/ijms25126434. Int J Mol Sci. 2024. PMID: 38928140 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

