Hexosamine pathway activation improves memory but does not extend lifespan in mice
- PMID: 36124412
- PMCID: PMC9577955
- DOI: 10.1111/acel.13711
Hexosamine pathway activation improves memory but does not extend lifespan in mice
Abstract
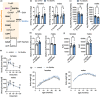
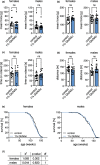
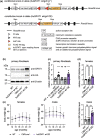
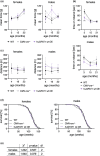
Glucosamine feeding and genetic activation of the hexosamine biosynthetic pathway (HBP) have been linked to improved protein quality control and lifespan extension. However, as an energy sensor, the HBP has been implicated in tumor progression and diabetes. Given these opposing outcomes, it is imperative to explore the long-term effects of chronic HBP activation in mammals. Thus, we asked if HBP activation affects metabolism, coordination, memory, and survival in mice. N-acetyl-D-glucosamine (GlcNAc) supplementation in the drinking water had no adverse effect on weight in males but increased weight in young females. Glucose or insulin tolerance was not affected up to 20 months of age. Of note, we observed improved memory in young male mice supplemented with GlcNAc. Survival was not changed by GlcNAc treatment. To assess the effects of genetic HBP activation, we overexpressed the pathway's key enzyme GFAT1 and a constitutively activated mutant form in all mouse tissues. We detected elevated levels of the HBP product UDP-GlcNAc in mouse brains, but did not find any effects on behavior, memory, or survival. Together, while dietary GlcNAc supplementation did not extend survival in mice, it positively affected memory and is generally well tolerated.
Keywords: GFAT1; hexosamine biosynthetic pathway; memory; metabolism; mouse survival.
© 2022 The Authors. Aging Cell published by Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no potential conflict of interests.
Figures

Similar articles
-
The Hexosamine Biosynthesis Pathway: Regulation and Function.Genes (Basel). 2023 Apr 18;14(4):933. doi: 10.3390/genes14040933. Genes (Basel). 2023. PMID: 37107691 Free PMC article. Review.
-
First characterization of glucose flux through the hexosamine biosynthesis pathway (HBP) in ex vivo mouse heart.J Biol Chem. 2020 Feb 14;295(7):2018-2033. doi: 10.1074/jbc.RA119.010565. Epub 2020 Jan 8. J Biol Chem. 2020. PMID: 31915250 Free PMC article.
-
Anticancer Properties of Hexosamine Analogs Designed to Attenuate Metabolic Flux through the Hexosamine Biosynthetic Pathway.ACS Chem Biol. 2023 Jan 20;18(1):151-165. doi: 10.1021/acschembio.2c00784. Epub 2023 Jan 10. ACS Chem Biol. 2023. PMID: 36626752
-
Dynamic actions of glucose and glucosamine on hexosamine biosynthesis in isolated adipocytes: differential effects on glucosamine 6-phosphate, UDP-N-acetylglucosamine, and ATP levels.J Biol Chem. 2004 Aug 20;279(34):35313-9. doi: 10.1074/jbc.M404133200. Epub 2004 Jun 15. J Biol Chem. 2004. PMID: 15199059
-
Dysregulation of hexosamine biosynthetic pathway wiring metabolic signaling circuits in cancer.Biochim Biophys Acta Gen Subj. 2023 Jan;1867(1):130250. doi: 10.1016/j.bbagen.2022.130250. Epub 2022 Oct 10. Biochim Biophys Acta Gen Subj. 2023. PMID: 36228878 Review.
Cited by
-
The Hexosamine Biosynthesis Pathway: Regulation and Function.Genes (Basel). 2023 Apr 18;14(4):933. doi: 10.3390/genes14040933. Genes (Basel). 2023. PMID: 37107691 Free PMC article. Review.
-
Nutrition Strategies Promoting Healthy Aging: From Improvement of Cardiovascular and Brain Health to Prevention of Age-Associated Diseases.Nutrients. 2022 Dec 22;15(1):47. doi: 10.3390/nu15010047. Nutrients. 2022. PMID: 36615705 Free PMC article. Review.
References
-
- Baron, A. D. , Zhu, J. S. , Zhu, J. H. , Weldon, H. , Maianu, L. , & Garvey, W. T. (1995). Glucosamine induces insulin resistance in vivo by affecting GLUT 4 translocation in skeletal muscle: Implications for glucose toxicity. Journal of Clinical Investigation, 96(6), 2792–2801. 10.1172/JCI118349 - DOI - PMC - PubMed
-
- de La Rosa, A. , Olaso‐Gonzalez, G. , Garcia‐Dominguez, E. , Mastaloudis, A. , Hester, S. N. , Wood, S. M. , Gomez‐Cabrera, M. C. , & Viña, J. (2022). Glucosamine supplementation improves physical performance in trained mice. Medicine and Science in Sports and Exercise, 54(3), 466–474. 10.1249/MSS.0000000000002821 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

