Analysis of viral integration reveals new insights of oncogenic mechanism in HBV-infected intrahepatic cholangiocarcinoma and combined hepatocellular-cholangiocarcinoma
- PMID: 36123506
- PMCID: PMC9701178
- DOI: 10.1007/s12072-022-10419-3
Analysis of viral integration reveals new insights of oncogenic mechanism in HBV-infected intrahepatic cholangiocarcinoma and combined hepatocellular-cholangiocarcinoma
Abstract
Background: Integration of HBV DNA into the human genome could progressively contribute to hepatocarcinogenesis. Both intrahepatic cholangiocarcinoma (ICC) and combined hepatocellular-cholangiocarcinoma (CHC) are known to be associated with HBV infection. However, the integration of HBV and mechanism of HBV-induced carcinogenesis in ICC and CHC remains unclear.
Methods: 41 patients with ICC and 20 patients with CHC were recruited in the study. We conducted HIVID analysis on these 61 samples to identify HBV integration sites in both the tumor tissues and adjacent non-tumor liver tissues. To further explore the effect of HBV integration on gene alteration, we selected paired tumors and adjacent non-tumor liver tissues from 3 ICC and 4 CHC patients for RNA-seq and WGS.
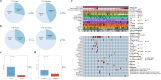
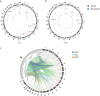
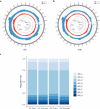
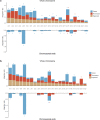
Results: We detected 493 HBV integration sites in ICC patients, of which 417 were from tumor samples and 76 were from non-tumor samples. And 246 HBV integration sites were detected in CHC patients, of which 156 were located in the genome of tumor samples and 90 were in non-tumor samples. Recurrent HBV integration events were detected in ICC including TERT, ZMAT4, MET, ANKFN1, PLXNB2, and in CHC like TERT, ALKBH5. Together with our established data of HBV-infected hepatocellular carcinoma, we found that HBV preferentially integrates into the specific regions which may affect the gene expression and regulation in cells and involved in carcinogenesis. We further performed genomic and transcriptomic sequencing of three ICC and four CHC patients, and found that HBV fragments could integrate near some important oncogene like TERT, causing large-scale genome variations on nearby genomic sequences, and at the same time changing the expression level of the oncogenes.
Conclusion: Comparative analysis demonstrates numerous newly discovered mutational events in ICC and CHC resulting from HBV insertions in the host genome. Our study provides an in-depth biological and clinical insights into HBV-induced ICC and CHC.
Keywords: Combined hepatocellular-cholangiocarcinoma; HBV integration; Hepatitis B virus; Intrahepatic cholangiocarcinoma.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Comprehensive characterization of viral integrations and genomic aberrations in HBV-infected intrahepatic cholangiocarcinomas.Hepatology. 2022 Apr;75(4):997-1011. doi: 10.1002/hep.32135. Epub 2021 Dec 5. Hepatology. 2022. PMID: 34478159
-
Whole-exome mutational and transcriptional landscapes of combined hepatocellular cholangiocarcinoma and intrahepatic cholangiocarcinoma reveal molecular diversity.Biochim Biophys Acta Mol Basis Dis. 2018 Jun;1864(6 Pt B):2360-2368. doi: 10.1016/j.bbadis.2018.01.027. Epub 2018 Feb 1. Biochim Biophys Acta Mol Basis Dis. 2018. PMID: 29408647
-
Genomic profiling of combined hepatocellular-cholangiocarcinoma reveals similar genetics to hepatocellular carcinoma.J Pathol. 2019 Jun;248(2):164-178. doi: 10.1002/path.5243. Epub 2019 Mar 8. J Pathol. 2019. PMID: 30690729
-
Combined hepatocellular cholangiocarcinoma: Controversies to be addressed.World J Gastroenterol. 2016 May 14;22(18):4459-65. doi: 10.3748/wjg.v22.i18.4459. World J Gastroenterol. 2016. PMID: 27182157 Free PMC article. Review.
-
Insights into the role of STAT3 in intrahepatic cholangiocarcinoma (Review).Mol Med Rep. 2022 May;25(5):171. doi: 10.3892/mmr.2022.12687. Epub 2022 Mar 18. Mol Med Rep. 2022. PMID: 35302174 Free PMC article. Review.
Cited by
-
HBV Infection and Host Interactions: The Role in Viral Persistence and Oncogenesis.Int J Mol Sci. 2023 Apr 21;24(8):7651. doi: 10.3390/ijms24087651. Int J Mol Sci. 2023. PMID: 37108816 Free PMC article. Review.
-
Mutational Landscape of Cholangiocarcinoma According to Different Etiologies: A Review.Cells. 2023 Apr 22;12(9):1216. doi: 10.3390/cells12091216. Cells. 2023. PMID: 37174616 Free PMC article. Review.
-
Genomic Analysis of Amphioxus Reveals a Wide Range of Fragments Homologous to Viral Sequences.Viruses. 2023 Mar 31;15(4):909. doi: 10.3390/v15040909. Viruses. 2023. PMID: 37112889 Free PMC article.
References
-
- Shafritz DA, Shouval D, Sherman HI, Hadziyannis SJ, Kew MC. Integration of hepatitis B virus DNA into the genome of liver cells in chronic liver disease and hepatocellular carcinoma. Studies in percutaneous liver biopsies and post-mortem tissue specimens. N Engl J Med. 1981;305:1067–1073. doi: 10.1056/NEJM198110293051807. - DOI - PubMed
-
- Mason WS, Gill US, Litwin S, Zhou Y, Peri S, Pop O, Hong ML, Naik S, Quaglia A, Bertoletti A, Kennedy PT. HBV DNA integration and clonal hepatocyte expansion in chronic hepatitis B patients considered immune tolerant. Gastroenterology. 2016;151:986–998.e984. doi: 10.1053/j.gastro.2016.07.012. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

