The changing view of insulin granule mobility: From conveyor belt to signaling hub
- PMID: 36120467
- PMCID: PMC9478610
- DOI: 10.3389/fendo.2022.983152
The changing view of insulin granule mobility: From conveyor belt to signaling hub
Abstract
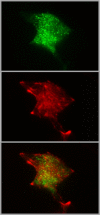
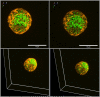
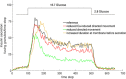
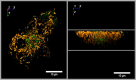
Before the advent of TIRF microscopy the fate of the insulin granule prior to secretion was deduced from biochemical investigations, electron microscopy and electrophysiological measurements. Since Calcium-triggered granule fusion is indisputably necessary to release insulin into the extracellular space, much effort was directed to the measure this event at the single granule level. This has also been the major application of the TIRF microscopy of the pancreatic beta cell when it became available about 20 years ago. To better understand the metabolic modulation of secretion, we were interested to characterize the entirety of the insulin granules which are localized in the vicinity of the plasma membrane to identify the characteristics which predispose to fusion. In this review we concentrate on how the description of granule mobility in the submembrane space has evolved as a result of progress in methodology. The granules are in a state of constant turnover with widely different periods of residence in this space. While granule fusion is associated +with prolonged residence and decreased lateral mobility, these characteristics may not only result from binding to the plasma membrane but also from binding to the cortical actin web, which is present in the immediate submembrane space. While granule age as such affects granule mobility and fusion probability, the preceding functional states of the beta cell leave their mark on these parameters, too. In summary, the submembrane granules form a highly dynamic heterogeneous population and contribute to the metabolic memory of the beta cells.
Keywords: TIRF microscopy; actin; calcium; insulin granules; insulin secretion; pancreatic islet.
Copyright © 2022 Gaus, Brüning, Groß, Müller and Rustenbeck.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Glucose but not KCl diminishes submembrane granule turnover in mouse beta-cells.J Mol Endocrinol. 2017 Oct;59(3):311-324. doi: 10.1530/JME-17-0063. Epub 2017 Aug 1. J Mol Endocrinol. 2017. PMID: 28765259
-
Bidirectional insulin granule turnover in the submembrane space during K(+) depolarization-induced secretion.Traffic. 2011 Sep;12(9):1166-78. doi: 10.1111/j.1600-0854.2011.01231.x. Epub 2011 Jul 13. Traffic. 2011. PMID: 21668594
-
Granule mobility, fusion frequency and insulin secretion are differentially affected by insulinotropic stimuli.Traffic. 2015 May;16(5):493-509. doi: 10.1111/tra.12261. Epub 2015 Feb 24. Traffic. 2015. PMID: 25615411
-
Insulin granule biogenesis, trafficking and exocytosis.Vitam Horm. 2009;80:473-506. doi: 10.1016/S0083-6729(08)00616-X. Vitam Horm. 2009. PMID: 19251047 Free PMC article. Review.
-
Insulin granule dynamics in pancreatic beta cells.Diabetologia. 2003 Aug;46(8):1029-45. doi: 10.1007/s00125-003-1153-1. Epub 2003 Jul 17. Diabetologia. 2003. PMID: 12879249 Review.
Cited by
-
The Dynamics of Calcium Signaling in Beta Cells-A Discussion on the Comparison of Experimental and Modelling Data.Int J Mol Sci. 2023 Feb 6;24(4):3206. doi: 10.3390/ijms24043206. Int J Mol Sci. 2023. PMID: 36834618 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

