BCL-2 inhibitor synergizes with PI3Kδ inhibitor and overcomes FLT3 inhibitor resistance in acute myeloid leukaemia
- PMID: 36119822
- PMCID: PMC9442011
BCL-2 inhibitor synergizes with PI3Kδ inhibitor and overcomes FLT3 inhibitor resistance in acute myeloid leukaemia
Abstract
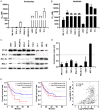
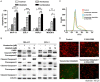
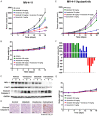
Inhibitors targeting the antiapoptotic molecule BCL-2 have therapeutic potential for the treatment of acute myeloid leukaemia (AML); however, BCL-2 inhibitors such as venetoclax exhibit limited monotherapy efficacy in relapsed or refractory human AML. PI3Kδ/AKT signalling has been shown to be constitutively active in AML patients. Here, we demonstrate that the combination of BCL-2 and PI3Kδ inhibitors exerts synergistic antitumour effects both in vitro and in vivo in AML. Cotreatment with venetoclax and the specific PI3Kδ inhibitor idelalisib significantly enhanced antiproliferative effects and induced caspase-dependent apoptosis in a panel of AML cell lines. The synergistic effects were mechanistically based on the inactivation of AKT/4E-BP-1 signalling and the reduction of MCL-1 expression, which diminished the binding of Bim to MCL-1. Notably, compared with the parental FLT3-ITD-positive MV-4-11, the acquired FLT3 inhibitor quizartinib-resistant xenograft model carrying the F691L mutation, exhibited a markedly higher sensitivity to venetoclax. Furthermore, venetoclax combined with idelalisib led to tumour regression in all animals in this quizartinib-resistant AML model. Thus, these data indicate that combined inhibition of BCL-2 and PI3Kδ may be a promising strategy in AML, especially for patients with FLT3-ITD and/or FLT3-TKD mutations.
Keywords: Acute myeloid leukaemia; BCL-2; FLT3; PI3Kδ; synergistic lethality.
AJCR Copyright © 2022.
Conflict of interest statement
None.
Figures


Similar articles
-
Combined inhibition of PI3Kδ and FLT3 signaling exerts synergistic antitumor activity and overcomes acquired drug resistance in FLT3-activated acute myeloid leukemia.Cancer Lett. 2018 Apr 28;420:49-59. doi: 10.1016/j.canlet.2018.01.071. Epub 2018 Feb 6. Cancer Lett. 2018. PMID: 29409989
-
Venetoclax combines synergistically with FLT3 inhibition to effectively target leukemic cells in FLT3-ITD+ acute myeloid leukemia models.Haematologica. 2021 Apr 1;106(4):1034-1046. doi: 10.3324/haematol.2019.244020. Haematologica. 2021. PMID: 32414851 Free PMC article.
-
FLT3 inhibition by olverembatinib (HQP1351) downregulates MCL-1 and synergizes with BCL-2 inhibitor lisaftoclax (APG-2575) in preclinical models of FLT3-ITD mutant acute myeloid leukemia.Transl Oncol. 2022 Jan;15(1):101244. doi: 10.1016/j.tranon.2021.101244. Epub 2021 Oct 25. Transl Oncol. 2022. PMID: 34710737 Free PMC article.
-
Quizartinib (AC220): a promising option for acute myeloid leukemia.Drug Des Devel Ther. 2019 Apr 8;13:1117-1125. doi: 10.2147/DDDT.S198950. eCollection 2019. Drug Des Devel Ther. 2019. PMID: 31114157 Free PMC article. Review.
-
Targeting Acute Myeloid Leukemia with Venetoclax; Biomarkers for Sensitivity and Rationale for Venetoclax-Based Combination Therapies.Cancers (Basel). 2022 Jul 15;14(14):3456. doi: 10.3390/cancers14143456. Cancers (Basel). 2022. PMID: 35884517 Free PMC article. Review.
Cited by
-
Targeting ST8SIA6-AS1 counteracts KRASG12C inhibitor resistance through abolishing the reciprocal activation of PLK1/c-Myc signaling.Exp Hematol Oncol. 2023 Dec 16;12(1):105. doi: 10.1186/s40164-023-00466-3. Exp Hematol Oncol. 2023. PMID: 38104151 Free PMC article.
-
Targeting Mcl-1 Degradation by Bergenin Inhibits Tumorigenesis of Colorectal Cancer Cells.Pharmaceuticals (Basel). 2023 Feb 6;16(2):241. doi: 10.3390/ph16020241. Pharmaceuticals (Basel). 2023. PMID: 37259388 Free PMC article.
-
The prognostic significance of genetics in acute myeloid leukemia under venetoclax-based treatment.Ann Hematol. 2024 Dec;103(12):5019-5033. doi: 10.1007/s00277-024-06050-x. Epub 2024 Oct 29. Ann Hematol. 2024. PMID: 39467855 Review.
References
-
- Short NJ, Konopleva M, Kadia TM, Borthakur G, Ravandi F, DiNardo CD, Daver N. Advances in the treatment of acute myeloid leukemia: new drugs and new challenges. Cancer Discov. 2020;10:506–525. - PubMed
-
- Khwaja A, Bjorkholm M, Gale RE, Levine RL, Jordan CT, Ehninger G, Bloomfield CD, Estey E, Burnett A, Cornelissen JJ, Scheinberg DA, Bouscary D, Linch DC. Acute myeloid leukaemia. Nat Rev Dis Primers. 2016;2:16010. - PubMed
-
- Döhner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Büchner T, Dombret H, Ebert BL, Fenaux P, Larson RA, Levine RL, Lo-Coco F, Naoe T, Niederwieser D, Ossenkoppele GJ, Sanz M, Sierra J, Tallman MS, Tien HF, Wei AH, Löwenberg B, Bloomfield CD. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129:424–447. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
