Amyotrophic lateral sclerosis
- PMID: 36116464
- PMCID: PMC10089700
- DOI: 10.1016/S0140-6736(22)01272-7
Amyotrophic lateral sclerosis
Abstract
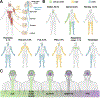
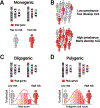
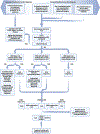
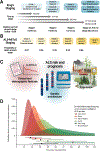
Amyotrophic lateral sclerosis is a fatal CNS neurodegenerative disease. Despite intensive research, current management of amyotrophic lateral sclerosis remains suboptimal from diagnosis to prognosis. Recognition of the phenotypic heterogeneity of amyotrophic lateral sclerosis, global CNS dysfunction, genetic architecture, and development of novel diagnostic criteria is clarifying the spectrum of clinical presentation and facilitating diagnosis. Insights into the pathophysiology of amyotrophic lateral sclerosis, identification of disease biomarkers and modifiable risks, along with new predictive models, scales, and scoring systems, and a clinical trial pipeline of mechanism-based therapies, are changing the prognostic landscape. Although most recent advances have yet to translate into patient benefit, the idea of amyotrophic lateral sclerosis as a complex syndrome is already having tangible effects in the clinic. This Seminar will outline these insights and discuss the status of the management of amyotrophic lateral sclerosis for the general neurologist, along with future prospects that could improve care and outcomes for patients with amyotrophic lateral sclerosis.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests ELF and SAG have a patent issued (US20200253977A1). SAG reports personal fees from Biogen, ITF Pharma, and Watermark, outside the submitted work. SP reports grants from the German Neuromuscular Society, the German-Israeli Foundation for Scientific Research and Development (GIF), and personal fees from Cytokinetics, Desitin Pharma, Italfarmaco, Biogen, Roche, and Zambon outside the submitted work. PJS reports consultancy and advisory board membership with Biogen, Benevolent AI, QurALIS, Quell, and Aclipse Therapeutics, outside the submitted work. GS reports personal fees from Mitsubishi Tanabe Pharma Corporation, Cyberdyne, Biogen Japan, Takeda Pharmaceutical, Nihon Pharmaceutical, and Teijin Pharma, outside the submitted work. LM and MGS declare no competing interests.
Figures
Similar articles
-
Emerging insights into the complex genetics and pathophysiology of amyotrophic lateral sclerosis.Lancet Neurol. 2022 May;21(5):465-479. doi: 10.1016/S1474-4422(21)00414-2. Epub 2022 Mar 22. Lancet Neurol. 2022. PMID: 35334234 Free PMC article. Review.
-
Biomarkers for amyotrophic lateral sclerosis.Curr Opin Neurol. 2022 Oct 1;35(5):699-704. doi: 10.1097/WCO.0000000000001094. Epub 2022 Aug 4. Curr Opin Neurol. 2022. PMID: 35942674 Review.
-
Recent advances in the diagnosis and prognosis of amyotrophic lateral sclerosis.Lancet Neurol. 2022 May;21(5):480-493. doi: 10.1016/S1474-4422(21)00465-8. Epub 2022 Mar 22. Lancet Neurol. 2022. PMID: 35334233 Free PMC article. Review.
-
Biofluid Biomarkers in the Prognosis of Amyotrophic Lateral Sclerosis: Recent Developments and Therapeutic Applications.Cells. 2023 Apr 18;12(8):1180. doi: 10.3390/cells12081180. Cells. 2023. PMID: 37190090 Free PMC article. Review.
-
Amyotrophic lateral sclerosis: recent advances and future therapies.Curr Opin Neurol. 2005 Dec;18(6):712-9. doi: 10.1097/01.wco.0000187248.21103.c5. Curr Opin Neurol. 2005. PMID: 16280684 Review.
Cited by
-
KIF1A, R1457Q, and P1688L Mutations Induce Protein Abnormal Aggregation and Autophagy Impairment in iPSC-Derived Motor Neurons.Biomedicines. 2024 Jul 30;12(8):1693. doi: 10.3390/biomedicines12081693. Biomedicines. 2024. PMID: 39200158 Free PMC article.
-
Advance directives in amyotrophic lateral sclerosis - a systematic review and meta-analysis.BMC Palliat Care. 2024 Jul 29;23(1):191. doi: 10.1186/s12904-024-01524-1. BMC Palliat Care. 2024. PMID: 39075493 Free PMC article.
-
Voice signals database of ALS patients with different dysarthria severity and healthy controls.Sci Data. 2024 Jul 19;11(1):800. doi: 10.1038/s41597-024-03597-2. Sci Data. 2024. PMID: 39030186 Free PMC article.
-
Immune-mediated diseases are associated with a higher risk of ALS incidence: a prospective cohort study from the UK Biobank.Front Immunol. 2024 Mar 5;15:1356132. doi: 10.3389/fimmu.2024.1356132. eCollection 2024. Front Immunol. 2024. PMID: 38504981 Free PMC article.
-
Brain Vascular Health in ALS Is Mediated through Motor Cortex Microvascular Integrity.Cells. 2023 Mar 21;12(6):957. doi: 10.3390/cells12060957. Cells. 2023. PMID: 36980297 Free PMC article. Review.
References
-
- Marin B, Fontana A, Arcuti S, Copetti M, Boumédiene F, Couratier P, et al. Age-specific ALS incidence: a dose-response meta-analysis. Eur J Epidemiol. 2018;33(7):621–34. - PubMed
-
- Luna J, Diagana M, Ait Aissa L, Tazir M, Ali Pacha L, Kacem I, et al. Clinical features and prognosis of amyotrophic lateral sclerosis in Africa: the TROPALS study. J Neurol Neurosurg Psychiatry. 2019;90(1):20–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

