Canine Idiopathic Arteriopathy, Appendicular Bone Infarcts, and Neoplastic Transformation of Bone Infarcts in 108 Dogs (Canis lupus familiaris)
- PMID: 36113969
- PMCID: PMC9827601
- DOI: 10.30802/AALAS-CM-22-000037
Canine Idiopathic Arteriopathy, Appendicular Bone Infarcts, and Neoplastic Transformation of Bone Infarcts in 108 Dogs (Canis lupus familiaris)
Abstract
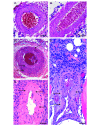
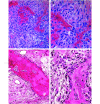
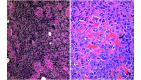
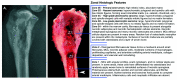
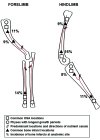
Osteosarcoma (OSA) is the most common primary bone tumor in both dogs and humans. The dog is an important research model for OSA, yet dogs have much higher prevalence of bone tumors than do humans, a disparity that has yet to be explained. Neoplastic transformation of cells within or adjacent to bone infarcts into primary bone tumors has been described in humans but only sparsely characterized in the veterinary literature. In this study, 653 cases of canine bone infarcts were received through a referral veterinary osteopathology service over a 14-y period. We identified an idiopathic disorder affecting the nutrient artery, termed canine idiopathic arteriopathy (CIA), which to our knowledge has no direct counterpart in human medicine. This disorder was documented alongside ischemic necrosis of the medullary cavity in 114 bone infarcts in 108 dogs. We hypothesize that CIA precipitated an ischemic environment, resulting in development of a bone infarct down- stream of the abnormal artery. In 52% (59 of 114) of cases, bone infarcts demonstrated evidence of repair (termed reparative bone infarcts [RBI]), while in 48% (55 of 114) of infarcts, a bone tumor was also present, including pleomorphic sarcoma, OSA, fibrosarcoma, and chondrosarcoma. In some cases, a spectrum of tumors was present. We hypothesize that the ischemic infarct environment provoked bone marrow mesenchymal stem cells (MSCs) to attempt repair of the stroma, and in approximately half of cases, MSCs underwent neoplastic transformation (BINT) to produce tumors. The most common sites of bone infarcts were the distal femur, distal radius, proximal humerus, and distal tibia, coinciding with common sites of canine OSA. The authors propose that CIA leading to bone infarcts and infarct-derived tumors, in combination with possible underdiagnosis of canine bone infarcts and misdiagnosis of some RBI as neoplasia, may contribute to the higher reported proportion of bone tumors in dogs compared with humans.
Figures


Similar articles
-
Osteocalcin and Osteonectin Expression in Canine Osteosarcoma.Vet Pathol. 2016 Jul;53(4):781-7. doi: 10.1177/0300985815626574. Epub 2016 Feb 29. Vet Pathol. 2016. PMID: 26926085
-
[Osteosarcoma of the distal femur, combined with multiple bone infarcts: the case of a 10-year-old poodle].Tierarztl Prax. 1988;16(3):303-6. Tierarztl Prax. 1988. PMID: 3187999 German.
-
HES1, a target of Notch signaling, is elevated in canine osteosarcoma, but reduced in the most aggressive tumors.BMC Vet Res. 2013 Jul 1;9:130. doi: 10.1186/1746-6148-9-130. BMC Vet Res. 2013. PMID: 23816051 Free PMC article.
-
Biology, diagnosis and treatment of canine appendicular osteosarcoma: similarities and differences with human osteosarcoma.Vet J. 2011 Sep;189(3):268-77. doi: 10.1016/j.tvjl.2010.08.014. Epub 2010 Oct 2. Vet J. 2011. PMID: 20889358 Review.
-
What do we know about canine osteosarcoma treatment? Review.Vet Res Commun. 2015 Mar;39(1):61-7. doi: 10.1007/s11259-014-9623-0. Epub 2014 Nov 26. Vet Res Commun. 2015. PMID: 25422073 Free PMC article. Review.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

