Neural signatures of auditory hypersensitivity following acoustic trauma
- PMID: 36111669
- PMCID: PMC9555866
- DOI: 10.7554/eLife.80015
Neural signatures of auditory hypersensitivity following acoustic trauma
Abstract
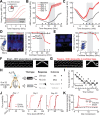
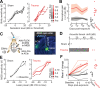
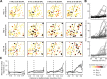
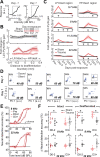
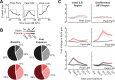
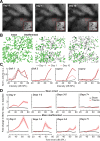
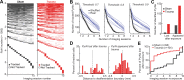
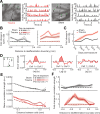
Neurons in sensory cortex exhibit a remarkable capacity to maintain stable firing rates despite large fluctuations in afferent activity levels. However, sudden peripheral deafferentation in adulthood can trigger an excessive, non-homeostatic cortical compensatory response that may underlie perceptual disorders including sensory hypersensitivity, phantom limb pain, and tinnitus. Here, we show that mice with noise-induced damage of the high-frequency cochlear base were behaviorally hypersensitive to spared mid-frequency tones and to direct optogenetic stimulation of auditory thalamocortical neurons. Chronic two-photon calcium imaging from ACtx pyramidal neurons (PyrNs) revealed an initial stage of spatially diffuse hyperactivity, hyper-correlation, and auditory hyperresponsivity that consolidated around deafferented map regions three or more days after acoustic trauma. Deafferented PyrN ensembles also displayed hypersensitive decoding of spared mid-frequency tones that mirrored behavioral hypersensitivity, suggesting that non-homeostatic regulation of cortical sound intensity coding following sensorineural loss may be an underlying source of auditory hypersensitivity. Excess cortical response gain after acoustic trauma was expressed heterogeneously among individual PyrNs, yet 40% of this variability could be accounted for by each cell's baseline response properties prior to acoustic trauma. PyrNs with initially high spontaneous activity and gradual monotonic intensity growth functions were more likely to exhibit non-homeostatic excess gain after acoustic trauma. This suggests that while cortical gain changes are triggered by reduced bottom-up afferent input, their subsequent stabilization is also shaped by their local circuit milieu, where indicators of reduced inhibition can presage pathological hyperactivity following sensorineural hearing loss.
Keywords: compensatory plasticity; excess central gain; hearing loss; homeostatic plasticity; hyperacusis; mouse; neuroscience; two-photon calcium imaging.
© 2022, McGill et al.
Conflict of interest statement
MM, AH, YW, AP, DC, KC, KH, AT, SK, DP No competing interests declared
Figures


Similar articles
-
A Role for KCNQ Channels on Cell Type-Specific Plasticity in Mouse Auditory Cortex after Peripheral Damage.J Neurosci. 2023 Mar 29;43(13):2277-2290. doi: 10.1523/JNEUROSCI.1070-22.2023. Epub 2023 Feb 22. J Neurosci. 2023. PMID: 36813573 Free PMC article.
-
The effects of acute and chronic noise trauma on stimulus-evoked activity across primary auditory cortex layers.J Neurophysiol. 2024 Feb 1;131(2):225-240. doi: 10.1152/jn.00427.2022. Epub 2024 Jan 10. J Neurophysiol. 2024. PMID: 38198658
-
Effects of lifetime noise exposure on the middle-age human auditory brainstem response, tinnitus and speech-in-noise intelligibility.Hear Res. 2018 Aug;365:36-48. doi: 10.1016/j.heares.2018.06.003. Epub 2018 Jun 12. Hear Res. 2018. PMID: 29913342
-
Advances in the neurobiology of hearing disorders: recent developments regarding the basis of tinnitus and hyperacusis.Prog Neurobiol. 2013 Dec;111:17-33. doi: 10.1016/j.pneurobio.2013.08.002. Epub 2013 Sep 6. Prog Neurobiol. 2013. PMID: 24012803 Review.
-
Auditory sensori-neural alterations induced by salicylate.Prog Neurobiol. 2000 Dec;62(6):583-631. doi: 10.1016/s0301-0082(00)00027-7. Prog Neurobiol. 2000. PMID: 10880852 Review.
Cited by
-
Map plasticity following noise exposure in auditory cortex of rats: implications for disentangling neural correlates of tinnitus and hyperacusis.Front Neurosci. 2024 May 31;18:1385942. doi: 10.3389/fnins.2024.1385942. eCollection 2024. Front Neurosci. 2024. PMID: 38881748 Free PMC article.
-
The human pupil and face encode sound affect and provide objective signatures of tinnitus and auditory hypersensitivity disorders.bioRxiv [Preprint]. 2024 Jan 18:2023.12.22.571929. doi: 10.1101/2023.12.22.571929. bioRxiv. 2024. PMID: 38187580 Free PMC article. Preprint.
-
Preservation of developmental spontaneous activity enables early auditory system maturation in deaf mice.PLoS Biol. 2023 Jun 27;21(6):e3002160. doi: 10.1371/journal.pbio.3002160. eCollection 2023 Jun. PLoS Biol. 2023. PMID: 37368868 Free PMC article.
-
Neuropeptide Y Signaling Regulates Recurrent Excitation in the Auditory Midbrain.J Neurosci. 2023 Nov 8;43(45):7626-7641. doi: 10.1523/JNEUROSCI.0900-23.2023. Epub 2023 Sep 13. J Neurosci. 2023. PMID: 37704372 Free PMC article.
-
Large-scale electrophysiology and deep learning reveal distorted neural signal dynamics after hearing loss.Elife. 2023 May 10;12:e85108. doi: 10.7554/eLife.85108. Elife. 2023. PMID: 37162188 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

