Bradykinin/bradykinin 1 receptor promotes brain microvascular endothelial cell permeability and proinflammatory cytokine release by downregulating Wnt3a
- PMID: 36111657
- PMCID: PMC10078380
- DOI: 10.1002/jbt.23213
Bradykinin/bradykinin 1 receptor promotes brain microvascular endothelial cell permeability and proinflammatory cytokine release by downregulating Wnt3a
Abstract
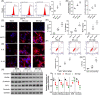
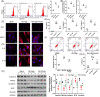
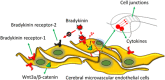
Stroke is a life-threatening disease with limited therapeutic options. Damage to the blood-brain barrier (BBB) is the key pathological feature of ischemic stroke. This study explored the role of the bradykinin (BK)/bradykinin 1 receptor (B1R) and its mechanism of action in the BBB. Human brain microvascular endothelial cells (BMECs) were used to test for cellular responses to BK by using the Cell Counting Kit-8 assay, 5-ethynyl-2'-deoxyuridine staining, enzyme-linked immunosorbent assay, flow cytometry, immunofluorescence, cellular permeability assays, and western blotting to evaluate cell viability, cytokine production, and reactive oxygen species (ROS) levels in vitro. A BBB induced by middle cerebral artery occlusion was used to evaluate BBB injuries, and the role played by BK/B1R in ischemic/reperfusion (I/R) was explored in a rat model. Results showed that BK reduced the viability of BMECs and increased the levels of proinflammatory cytokines (interleukin 6 [IL-6], IL-18, and monocyte chemoattractant protein-1) and ROS. Additionally, cellular permeability was increased by BK treatment, and the expression of tight junction proteins (claudin-5 and occludin) was decreased. Interestingly, Wnt3a expression was inhibited by BK and exogenous Wnt3a restored the effects of BK on BMECs. In an in vivo I/R rat model, knockdown of B1R significantly decreased infarct volume and inflammation in I/R rats. Our results suggest that BK might be a key inducer of BBB injury and B1R knockdown might provide a beneficial effect by upregulating Wnt3a.
Keywords: B1R; blood-brain barrier; bradykinin; inflammation; stroke.
© 2022 The Authors. Journal of Biochemical and Molecular Toxicology published by Wiley Periodicals LLC.
Conflict of interest statement
All authors declare no conflict of interest.
Figures



Similar articles
-
Bradykinin-bradykinin receptor (B1R) signalling is involved in the blood-brain barrier disruption in moyamoya disease.J Cell Mol Med. 2023 Dec;27(24):4069-4079. doi: 10.1111/jcmm.17989. Epub 2023 Oct 11. J Cell Mol Med. 2023. PMID: 37818853 Free PMC article.
-
Neurolysin substrates bradykinin, neurotensin and substance P enhance brain microvascular permeability in a human in vitro model.J Neuroendocrinol. 2021 Feb;33(2):e12931. doi: 10.1111/jne.12931. Epub 2021 Jan 28. J Neuroendocrinol. 2021. PMID: 33506602 Free PMC article.
-
Bradykinin regulates the expression of claudin-5 in brain microvascular endothelial cells via calcium-induced calcium release.J Neurosci Res. 2014 May;92(5):597-606. doi: 10.1002/jnr.23350. Epub 2014 Jan 27. J Neurosci Res. 2014. PMID: 24464430
-
Vasomotor and permeability effects of bradykinin in the cerebral microcirculation.Immunopharmacology. 1996 Jun;33(1-3):257-63. doi: 10.1016/0162-3109(96)00068-9. Immunopharmacology. 1996. PMID: 8856159 Review.
-
The development of the bradykinin agonist labradimil as a means to increase the permeability of the blood-brain barrier: from concept to clinical evaluation.Clin Pharmacokinet. 2001;40(2):105-23. doi: 10.2165/00003088-200140020-00003. Clin Pharmacokinet. 2001. PMID: 11286321 Review.
Cited by
-
Bradykinin-bradykinin receptor (B1R) signalling is involved in the blood-brain barrier disruption in moyamoya disease.J Cell Mol Med. 2023 Dec;27(24):4069-4079. doi: 10.1111/jcmm.17989. Epub 2023 Oct 11. J Cell Mol Med. 2023. PMID: 37818853 Free PMC article.
-
Glutathione in HIV-Associated Neurocognitive Disorders.Curr Issues Mol Biol. 2024 May 31;46(6):5530-5549. doi: 10.3390/cimb46060330. Curr Issues Mol Biol. 2024. PMID: 38921002 Free PMC article. Review.
-
Multiple Sclerosis: Inflammatory and Neuroglial Aspects.Curr Issues Mol Biol. 2023 Feb 8;45(2):1443-1470. doi: 10.3390/cimb45020094. Curr Issues Mol Biol. 2023. PMID: 36826039 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

