Directing the Way-Receptor and Chemical Targeting Strategies for Nucleic Acid Delivery
- PMID: 36109461
- PMCID: PMC9483255
- DOI: 10.1007/s11095-022-03385-w
Directing the Way-Receptor and Chemical Targeting Strategies for Nucleic Acid Delivery
Abstract
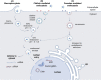
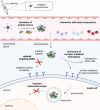
Nucleic acid therapeutics have shown great potential for the treatment of numerous diseases, such as genetic disorders, cancer and infections. Moreover, they have been successfully used as vaccines during the COVID-19 pandemic. In order to unfold full therapeutical potential, these nano agents have to overcome several barriers. Therefore, directed transport to specific tissues and cell types remains a central challenge to receive carrier systems with enhanced efficiency and desired biodistribution profiles. Active targeting strategies include receptor-targeting, mediating cellular uptake based on ligand-receptor interactions, and chemical targeting, enabling cell-specific delivery as a consequence of chemically and structurally modified carriers. With a focus on synthetic delivery systems including polyplexes, lipid-based systems such as lipoplexes and lipid nanoparticles, and direct conjugates optimized for various types of nucleic acids (DNA, mRNA, siRNA, miRNA, oligonucleotides), we highlight recent achievements, exemplified by several nucleic acid drugs on the market, and discuss challenges for targeted delivery to different organs such as brain, eye, liver, lung, spleen and muscle in vivo.
Keywords: lipoplex; pDNA; polyplex; siRNA; targeting.
© 2022. The Author(s).
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures




Similar articles
-
Targeting nucleic acid-based therapeutics to tumors: Challenges and strategies for polyplexes.J Control Release. 2022 Jun;346:110-135. doi: 10.1016/j.jconrel.2022.04.013. Epub 2022 Apr 21. J Control Release. 2022. PMID: 35436520 Review.
-
Lipid-based nucleic acid therapeutics with in vivo efficacy.Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2023 Mar;15(2):e1856. doi: 10.1002/wnan.1856. Epub 2022 Sep 30. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2023. PMID: 36180107 Free PMC article. Review.
-
Nonviral pulmonary delivery of siRNA.Acc Chem Res. 2012 Jul 17;45(7):961-70. doi: 10.1021/ar200110p. Epub 2011 Sep 9. Acc Chem Res. 2012. PMID: 21905687
-
Advances in modification and delivery of nucleic acid drugs.Zhejiang Da Xue Xue Bao Yi Xue Ban. 2023 Aug 25;52(4):417-428. doi: 10.3724/zdxbyxb-2023-0130. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2023. PMID: 37643976 Free PMC article. Review. Chinese, English.
-
Post-PEGylation of siRNA Lipo-oligoamino Amide Polyplexes Using Tetra-glutamylated Folic Acid as Ligand for Receptor-Targeted Delivery.Mol Pharm. 2016 Jul 5;13(7):2332-45. doi: 10.1021/acs.molpharmaceut.6b00102. Epub 2016 May 31. Mol Pharm. 2016. PMID: 27177200
Cited by
-
Dynamic carriers for therapeutic RNA delivery.Proc Natl Acad Sci U S A. 2024 Mar 12;121(11):e2307799120. doi: 10.1073/pnas.2307799120. Epub 2024 Mar 4. Proc Natl Acad Sci U S A. 2024. PMID: 38437544 Free PMC article.
-
Nanodrug Delivery Systems for Myasthenia Gravis: Advances and Perspectives.Pharmaceutics. 2024 May 11;16(5):651. doi: 10.3390/pharmaceutics16050651. Pharmaceutics. 2024. PMID: 38794313 Free PMC article. Review.
-
Nucleic Acid Delivery.Pharm Res. 2023 Jan;40(1):1-2. doi: 10.1007/s11095-023-03476-2. Pharm Res. 2023. PMID: 36735107 Free PMC article. No abstract available.
-
Navigating the intricate in-vivo journey of lipid nanoparticles tailored for the targeted delivery of RNA therapeutics: a quality-by-design approach.J Nanobiotechnology. 2024 Nov 14;22(1):710. doi: 10.1186/s12951-024-02972-w. J Nanobiotechnology. 2024. PMID: 39543630 Free PMC article. Review.
-
Extracellular Vesicles for Therapeutic Nucleic Acid Delivery: Loading Strategies and Challenges.Int J Mol Sci. 2023 Apr 14;24(8):7287. doi: 10.3390/ijms24087287. Int J Mol Sci. 2023. PMID: 37108446 Free PMC article. Review.
References
-
- Gene Therapy Clinical Trials Worldwide. 09.05.2022. Available from: https://a873679.fmphost.com/fmi/webd/GTCT.
-
- Mulligan RC. The basic science of gene therapy. Science. 1993;260(5110):926–932. - PubMed
-
- Friedmann T, Roblin R. Gene therapy for human genetic disease? Science. 1972;175(4025):949–955. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

