Immune tolerance against infused FVIII in hemophilia A is mediated by PD-L1+ Tregs
- PMID: 36107620
- PMCID: PMC9663153
- DOI: 10.1172/JCI159925
Immune tolerance against infused FVIII in hemophilia A is mediated by PD-L1+ Tregs
Abstract
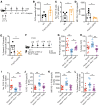
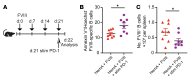
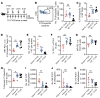
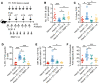
A major complication of hemophilia A therapy is the development of alloantibodies (inhibitors) that neutralize intravenously administered coagulation factor VIII (FVIII). Immune tolerance induction therapy (ITI) by repetitive FVIII injection can eradicate inhibitors, and thereby reduce morbidity and treatment costs. However, ITI success is difficult to predict and the underlying immunological mechanisms are unknown. Here, we demonstrated that immune tolerance against FVIII under nonhemophilic conditions was maintained by programmed death (PD) ligand 1-expressing (PD-L1-expressing) regulatory T cells (Tregs) that ligated PD-1 on FVIII-specific B cells, causing them to undergo apoptosis. FVIII-deficient mice injected with FVIII lacked such Tregs and developed inhibitors. Using an ITI mouse model, we found that repetitive FVIII injection induced FVIII-specific PD-L1+ Tregs and reengaged removal of inhibitor-forming B cells. We also demonstrated the existence of FVIII-specific Tregs in humans and showed that such Tregs upregulated PD-L1 in patients with hemophilia after successful ITI. Simultaneously, FVIII-specific B cells upregulated PD-1 and became killable by Tregs. In summary, we showed that PD-1-mediated B cell tolerance against FVIII operated in healthy individuals and in patients with hemophilia A without inhibitors, and that ITI reengaged this mechanism. These findings may impact monitoring of ITI success and treatment of patients with hemophilia A.
Keywords: Cellular immune response; Coagulation; Hematology; Immunology; Immunotherapy.
Figures

Comment in
-
Until programmed death do us tolerant.J Clin Invest. 2022 Nov 15;132(22):e164858. doi: 10.1172/JCI164858. J Clin Invest. 2022. PMID: 36377659 Free PMC article.
-
Germline variants of the immune checkpoint proteins PD-1, PD-l1 and CTLA-4 and immune tolerance induction outcome in patients with inherited haemophilia A.Haemophilia. 2023 Sep;29(5):1366-1368. doi: 10.1111/hae.14823. Epub 2023 Jul 6. Haemophilia. 2023. PMID: 37410805 No abstract available.
Similar articles
-
Review of immune tolerance induction in hemophilia A.Blood Rev. 2018 Jul;32(4):326-338. doi: 10.1016/j.blre.2018.02.003. Epub 2018 Feb 15. Blood Rev. 2018. PMID: 29482894 Review.
-
Escape or Fight: Inhibitors in Hemophilia A.Front Immunol. 2020 Mar 24;11:476. doi: 10.3389/fimmu.2020.00476. eCollection 2020. Front Immunol. 2020. PMID: 32265927 Free PMC article. Review.
-
Long-term tolerance to factor VIII is achieved by administration of interleukin-2/interleukin-2 monoclonal antibody complexes and low dosages of factor VIII.J Thromb Haemost. 2014 Jun;12(6):921-31. doi: 10.1111/jth.12576. J Thromb Haemost. 2014. PMID: 24684505 Free PMC article.
-
New protocol for immune tolerance induction in acquired hemophilia.Haematologica. 2000 Oct;85(10 Suppl):64-8. Haematologica. 2000. PMID: 11187874
-
B Cell Depletion Eliminates FVIII Memory B Cells and Enhances AAV8-coF8 Immune Tolerance Induction When Combined With Rapamycin.Front Immunol. 2020 Jun 24;11:1293. doi: 10.3389/fimmu.2020.01293. eCollection 2020. Front Immunol. 2020. PMID: 32670285 Free PMC article.
Cited by
-
Targeted delivery of Fc-fused PD-L1 for effective management of acute and chronic colitis.Nat Commun. 2024 Feb 23;15(1):1673. doi: 10.1038/s41467-024-46025-0. Nat Commun. 2024. PMID: 38396052 Free PMC article.
-
Prediction of the chance of successful immune tolerance induction in persons with severe hemophilia A and inhibitors: a clinical prediction model.Res Pract Thromb Haemost. 2024 Oct 3;8(7):102580. doi: 10.1016/j.rpth.2024.102580. eCollection 2024 Oct. Res Pract Thromb Haemost. 2024. PMID: 39558913 Free PMC article.
-
Abnormal frequency of the memory B cell subsets and plasmablasts in patients with congenital severe hemophilia A: correlation with "Inhibitor" formation.Blood Res. 2024 Apr 16;59(1):16. doi: 10.1007/s44313-024-00017-7. Blood Res. 2024. PMID: 38625415 Free PMC article.
-
Until programmed death do us tolerant.J Clin Invest. 2022 Nov 15;132(22):e164858. doi: 10.1172/JCI164858. J Clin Invest. 2022. PMID: 36377659 Free PMC article.
-
Hemophilia a patients with inhibitors: Mechanistic insights and novel therapeutic implications.Front Immunol. 2022 Dec 8;13:1019275. doi: 10.3389/fimmu.2022.1019275. eCollection 2022. Front Immunol. 2022. PMID: 36569839 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

