Microglia subtypes show substrate- and time-dependent phagocytosis preferences and phenotype plasticity
- PMID: 36105813
- PMCID: PMC9465456
- DOI: 10.3389/fimmu.2022.945485
Microglia subtypes show substrate- and time-dependent phagocytosis preferences and phenotype plasticity
Abstract
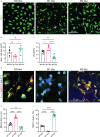
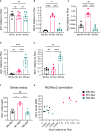
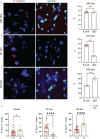
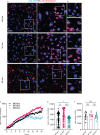
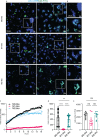
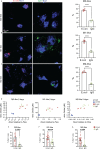
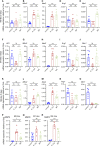
Microglia are phagocytosis-competent CNS cells comprising a spectrum of subtypes with beneficial and/or detrimental functions in acute and chronic neurodegenerative disorders. The heterogeneity of microglia suggests differences in phagocytic activity and phenotype plasticity between microglia subtypes. To study these issues, primary murine glial cultures were cultivated in the presence of serum, different growth factors and cytokines to obtain M0-like, M1-like, and M2-like microglia as confirmed by morphology, M1/M2 gene marker expression, and nitric oxide assay. Single-cell analysis after 3 hours of phagocytosis of E.coli particles or IgG-opsonized beads showed equal internalization by M0-like microglia, whereas M1-like microglia preferably internalized E.coli particles and M2-like microglia preferably internalized IgG beads, suggesting subtype-specific preferences for different phagocytosis substrates. Time-lapse live-cells imaging over 16 hours revealed further differences between microglia subtypes in phagocytosis preference and internalization dynamics. M0- and, more efficiently, M1-like microglia continuously internalized E.coli particles for 16 hours, whereas M2-like microglia discontinued internalization after approximately 8 hours. IgG beads were continuously internalized by M0- and M1-like microglia but strikingly less by M2-like microglia. M2-like microglia initially showed continuous internalization similar to M0-like microglia but again discontinuation of internalization after 8 hours suggesting that the time of substrate exposure differently affect microglia subtypes. After prolonged exposure to E.coli particles or IgG beads for 5 days all microglia subtypes showed increased internalization of E.coli particles compared to IgG beads, increased nitric oxide release and up-regulation of M1 gene markers, irrespectively of the phagocytosis substrate, suggesting phenotype plasticity. In summary, microglia subtypes show substrate- and time-dependent phagocytosis preferences and phenotype plasticity. The results suggest that prolonged phagocytosis substrate exposure enhances M1-like profiles and M2-M1 repolarization of microglia. Similar processes may also take place in conditions of acute and chronic brain insults when microglia encounter different types of phagocytic substrates.
Keywords: brain; immune response; inflammation; live imaging; microglia; phagocytosis; plasticity; polarization.
Copyright © 2022 Li, Wernersbach, Harms and Schäfer.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Oxymatrine inhibits neuroinflammation byRegulating M1/M2 polarization in N9 microglia through the TLR4/NF-κB pathway.Int Immunopharmacol. 2021 Nov;100:108139. doi: 10.1016/j.intimp.2021.108139. Epub 2021 Sep 10. Int Immunopharmacol. 2021. PMID: 34517275
-
The potential roles of m6A modification in regulating the inflammatory response in microglia.J Neuroinflammation. 2021 Jul 5;18(1):149. doi: 10.1186/s12974-021-02205-z. J Neuroinflammation. 2021. PMID: 34225746 Free PMC article.
-
The plasticity of primary microglia and their multifaceted effects on endogenous neural stem cells in vitro and in vivo.J Neuroinflammation. 2018 Aug 13;15(1):226. doi: 10.1186/s12974-018-1261-y. J Neuroinflammation. 2018. PMID: 30103769 Free PMC article.
-
Diversity and plasticity of microglial cells in psychiatric and neurological disorders.Pharmacol Ther. 2015 Oct;154:21-35. doi: 10.1016/j.pharmthera.2015.06.010. Epub 2015 Jun 27. Pharmacol Ther. 2015. PMID: 26129625 Review.
-
A richer and more diverse future for microglia phenotypes.Heliyon. 2023 Mar 21;9(4):e14713. doi: 10.1016/j.heliyon.2023.e14713. eCollection 2023 Apr. Heliyon. 2023. PMID: 37025898 Free PMC article. Review.
Cited by
-
Glioblastoma-instructed microglia transition to heterogeneous phenotypic states with phagocytic and dendritic cell-like features in patient tumors and patient-derived orthotopic xenografts.bioRxiv [Preprint]. 2023 Dec 12:2023.03.05.531162. doi: 10.1101/2023.03.05.531162. bioRxiv. 2023. Update in: Genome Med. 2024 Apr 2;16(1):51. doi: 10.1186/s13073-024-01321-8 PMID: 36945572 Free PMC article. Updated. Preprint.
-
Role of α-synuclein in microglia: autophagy and phagocytosis balance neuroinflammation in Parkinson's disease.Inflamm Res. 2023 Mar;72(3):443-462. doi: 10.1007/s00011-022-01676-x. Epub 2023 Jan 4. Inflamm Res. 2023. PMID: 36598534 Review.
-
Macrophage/Microglia Sirt3 Contributes to the Anti-inflammatory Effects of Resveratrol Against Experimental Intracerebral Hemorrhage in Mice.Cell Mol Neurobiol. 2023 Aug;43(6):2871-2882. doi: 10.1007/s10571-023-01325-9. Epub 2023 Feb 14. Cell Mol Neurobiol. 2023. PMID: 36786945
-
Glioblastoma-instructed microglia transition to heterogeneous phenotypic states with phagocytic and dendritic cell-like features in patient tumors and patient-derived orthotopic xenografts.Genome Med. 2024 Apr 2;16(1):51. doi: 10.1186/s13073-024-01321-8. Genome Med. 2024. PMID: 38566128 Free PMC article.
-
Immune response of BV-2 microglial cells is impacted by peroxisomal beta-oxidation.Front Mol Neurosci. 2023 Dec 18;16:1299314. doi: 10.3389/fnmol.2023.1299314. eCollection 2023. Front Mol Neurosci. 2023. PMID: 38164407 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

