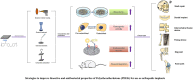
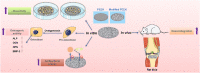
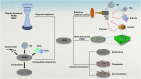
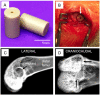
Strategies to improve bioactive and antibacterial properties of polyetheretherketone (PEEK) for use as orthopedic implants
- PMID: 36105676
- PMCID: PMC9466655
- DOI: 10.1016/j.mtbio.2022.100402
Strategies to improve bioactive and antibacterial properties of polyetheretherketone (PEEK) for use as orthopedic implants
Abstract
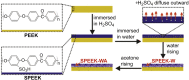
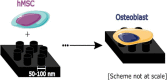
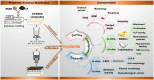
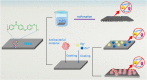
Polyetheretherketone (PEEK) has gradually become the mainstream material for preparing orthopedic implants due to its similar elastic modulus to human bone, high strength, excellent wear resistance, radiolucency, and biocompatibility. Since the 1990s, PEEK has increasingly been used in orthopedics. Yet, the widespread application of PEEK is limited by its bio-inertness, hydrophobicity, and susceptibility to microbial infections. Further enhancing the osteogenic properties of PEEK-based implants remains a difficult task. This article reviews some modification methods of PEEK in the last five years, including surface modification of PEEK or incorporating materials into the PEEK matrix. For surface modification, PEEK can be modified by chemical treatment, physical treatment, or surface coating with bioactive substances. For PEEK composite material, adding bioactive filler into PEEK through the melting blending method or 3D printing technology can increase the biological activity of PEEK. In addition, some modification methods such as sulfonation treatment of PEEK or grafting antibacterial substances on PEEK can enhance the antibacterial performance of PEEK. These strategies aim to improve the bioactive and antibacterial properties of the modified PEEK. The researchers believe that these modifications could provide valuable guidance on the future design of PEEK orthopedic implants.
Keywords: Antibacterial properties; Biocompatibility; Coating; Composite; Modification; Orthopedic implants; Polyetheretherketone.
© 2022 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

Similar articles
-
Strategies to improve the performance of polyetheretherketone (PEEK) as orthopedic implants: from surface modification to addition of bioactive materials.J Mater Chem B. 2024 May 15;12(19):4533-4552. doi: 10.1039/d3tb02740f. J Mater Chem B. 2024. PMID: 38477504 Review.
-
Approaches to Biofunctionalize Polyetheretherketone for Antibacterial: A Review.Front Bioeng Biotechnol. 2022 May 13;10:895288. doi: 10.3389/fbioe.2022.895288. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35646862 Free PMC article. Review.
-
Application of biomolecules modification strategies on PEEK and its composites for osteogenesis and antibacterial properties.Colloids Surf B Biointerfaces. 2022 Jul;215:112492. doi: 10.1016/j.colsurfb.2022.112492. Epub 2022 Apr 12. Colloids Surf B Biointerfaces. 2022. PMID: 35430485 Review.
-
Enhancing bioactivity and biocompatibility of polyetheretherketone (PEEK) for dental and maxillofacial implants: A novel sequential soaking process.Heliyon. 2024 Jun 23;10(13):e33381. doi: 10.1016/j.heliyon.2024.e33381. eCollection 2024 Jul 15. Heliyon. 2024. PMID: 39027560 Free PMC article.
-
State-of-the-art polyetheretherketone three-dimensional printing and multifunctional modification for dental implants.Front Bioeng Biotechnol. 2023 Oct 19;11:1271629. doi: 10.3389/fbioe.2023.1271629. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 37929192 Free PMC article. Review.
Cited by
-
Surface Treatments of PEEK for Osseointegration to Bone.Biomolecules. 2023 Mar 2;13(3):464. doi: 10.3390/biom13030464. Biomolecules. 2023. PMID: 36979399 Free PMC article. Review.
-
Polyetheretherketone implants with hierarchical porous structure for boosted osseointegration.Biomater Res. 2023 Jun 27;27(1):61. doi: 10.1186/s40824-023-00407-5. Biomater Res. 2023. PMID: 37370127 Free PMC article.
-
Recent Advances in the Application of Natural and Synthetic Polymer-Based Scaffolds in Musculoskeletal Regeneration.Polymers (Basel). 2022 Oct 27;14(21):4566. doi: 10.3390/polym14214566. Polymers (Basel). 2022. PMID: 36365559 Free PMC article. Review.
-
The ability of SPEEK to promote the proliferation and osteogenic differentiation of BMSCs on PEEK surfaces.Heliyon. 2024 Aug 18;10(16):e36448. doi: 10.1016/j.heliyon.2024.e36448. eCollection 2024 Aug 30. Heliyon. 2024. PMID: 39253123 Free PMC article.
-
Toward the Production of Hydroxyapatite/Poly(Ether-Ether-Ketone) (PEEK) Biocomposites: Exploring the Physicochemical, Mechanical, Cytotoxic and Antimicrobial Properties.Polymers (Basel). 2024 Sep 5;16(17):2520. doi: 10.3390/polym16172520. Polymers (Basel). 2024. PMID: 39274153 Free PMC article.
References
-
- Kokubo T., Kim H.-M., Kawashita M. Novel bioactive materials with different mechanical properties. Biomaterials. 2003;24(13):2161–2175. - PubMed
-
- Panayotov I.V., Orti V., Cuisinier F., Yachouh J. Polyetheretherketone (PEEK) for medical applications. J. Mater. Sci. Mater. Med. 2016;27(7):118. - PubMed
-
- Vadapalli S., Sairyo K., Goel V.K., Robon M., Biyani A., Khandha A., Ebraheim N.A. Biomechanical rationale for using polyetheretherketone (PEEK) spacers for lumbar interbody fusion-A finite element study. Spine. 2006;31(26):E992–E998. - PubMed
-
- Deng Y., Tang W., Li Z. Repairing a facial cleft by polyether-ether-ketone implant combined with titanium mesh. J. Craniofac. Surg. 2018;29(6):e582–e585. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

