Aroylhydrazone Glycoconjugate Prochelators Exploit Glucose Transporter 1 (GLUT1) to Target Iron in Cancer Cells
- PMID: 36105345
- PMCID: PMC9465708
- DOI: 10.1021/acsmedchemlett.2c00250
Aroylhydrazone Glycoconjugate Prochelators Exploit Glucose Transporter 1 (GLUT1) to Target Iron in Cancer Cells
Abstract
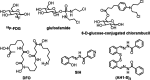
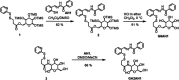
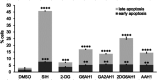
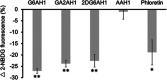
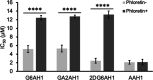
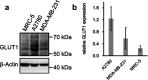
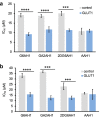
Glycoconjugation strategies in anticancer drug discovery exploit the high expression of glucose transporters in malignant cells to achieve preferential uptake and hence attractive pharmacological characteristics of increased therapeutic windows and decreased unwanted toxicity. Here we present the design of glycoconjugated prochelators of aroylhydrazone AH1, an antiproliferative scavenger that targets the increased iron demand of rapidly proliferating malignant cells. The constructs feature a monosaccharide (d-glucose, d-glucosamine, or glycolytic inhibitor 2-deoxy-d-glucose) connected at the C2 or C6 position via a short linker, which masks the chelator through a disulfide bond susceptible to intracellular reduction. Cellular assays showed that the glycoconjugates rely on the GLUT1 transporter for uptake, lead to intracellular iron deprivation, and present antiproliferative activity. Ectopic overexpression of GLUT1 in malignant and normal cells increased the uptake and toxicity of the glycoconjugated prochelators, demonstrating that these compounds are well suited for targeting cells overexpressing glucose transporters and therefore for selective iron sequestration in malignant cells.
© 2022 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
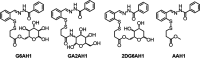


Similar articles
-
Thiol-Reactive Arylsulfonate Masks for Phenolate Donors in Antiproliferative Iron Prochelators.Inorg Chem. 2022 Dec 12;61(49):19974-19982. doi: 10.1021/acs.inorgchem.2c03250. Epub 2022 Dec 1. Inorg Chem. 2022. PMID: 36455205 Free PMC article.
-
Chemical Approach to Positional Isomers of Glucose-Platinum Conjugates Reveals Specific Cancer Targeting through Glucose-Transporter-Mediated Uptake in Vitro and in Vivo.J Am Chem Soc. 2016 Sep 28;138(38):12541-51. doi: 10.1021/jacs.6b06937. Epub 2016 Sep 16. J Am Chem Soc. 2016. PMID: 27570149 Free PMC article.
-
Targeting Iron in Colon Cancer via Glycoconjugation of Thiosemicarbazone Prochelators.Bioconjug Chem. 2016 Aug 17;27(8):1807-12. doi: 10.1021/acs.bioconjchem.6b00332. Epub 2016 Jul 29. Bioconjug Chem. 2016. PMID: 27471913 Free PMC article.
-
cypate-d: -(+)-glucosamine (cyp-GlcN), and d: -(+)-glucosamine-cypate-d: -(+)-glucosamine (cyp-2GlcN).2012 Aug 8 [updated 2012 Sep 13]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2012 Aug 8 [updated 2012 Sep 13]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 22993875 Free Books & Documents. Review.
-
Glycoconjugation as a Promising Treatment Strategy for Psoriasis.J Pharmacol Exp Ther. 2020 May;373(2):204-212. doi: 10.1124/jpet.119.263657. Epub 2020 Mar 10. J Pharmacol Exp Ther. 2020. PMID: 32156758 Review.
Cited by
-
Exploring a Gemcitabine-Glucose Hybrid as a Glycoconjugate Prodrug.ACS Omega. 2024 Jul 9;9(29):31703-31713. doi: 10.1021/acsomega.4c02417. eCollection 2024 Jul 23. ACS Omega. 2024. PMID: 39072123 Free PMC article.
-
Thiol-Reactive Arylsulfonate Masks for Phenolate Donors in Antiproliferative Iron Prochelators.Inorg Chem. 2022 Dec 12;61(49):19974-19982. doi: 10.1021/acs.inorgchem.2c03250. Epub 2022 Dec 1. Inorg Chem. 2022. PMID: 36455205 Free PMC article.
-
Quinoline-based tetrazolium prochelators: formazan release, iron sequestration, and antiproliferative efficacy in cancer cells.Chem Commun (Camb). 2024 Jun 11;60(48):6150-6153. doi: 10.1039/d4cc01523a. Chem Commun (Camb). 2024. PMID: 38804255
-
Design of Tetrazolium Cations for the Release of Antiproliferative Formazan Chelators in Mammalian Cells.J Am Chem Soc. 2023 Jul 19;145(28):15197-15206. doi: 10.1021/jacs.3c02033. Epub 2023 Jul 6. J Am Chem Soc. 2023. PMID: 37410992 Free PMC article.
-
Targeting iron to contrast cancer progression.Curr Opin Chem Biol. 2023 Jun;74:102315. doi: 10.1016/j.cbpa.2023.102315. Epub 2023 May 13. Curr Opin Chem Biol. 2023. PMID: 37187095 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

