Naturally derived indole alkaloids targeting regulated cell death (RCD) for cancer therapy: from molecular mechanisms to potential therapeutic targets
- PMID: 36104717
- PMCID: PMC9471064
- DOI: 10.1186/s13045-022-01350-z
Naturally derived indole alkaloids targeting regulated cell death (RCD) for cancer therapy: from molecular mechanisms to potential therapeutic targets
Erratum in
-
Correction: Naturally derived indole alkaloids targeting regulated cell death (RCD) for cancer therapy: from molecular mechanisms to potential therapeutic targets.J Hematol Oncol. 2022 Oct 24;15(1):151. doi: 10.1186/s13045-022-01371-8. J Hematol Oncol. 2022. PMID: 36280887 Free PMC article. No abstract available.
Abstract
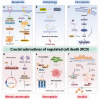
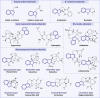
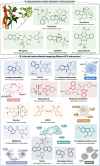
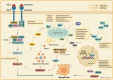
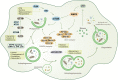
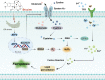
Regulated cell death (RCD) is a critical and active process that is controlled by specific signal transduction pathways and can be regulated by genetic signals or drug interventions. Meanwhile, RCD is closely related to the occurrence and therapy of multiple human cancers. Generally, RCD subroutines are the key signals of tumorigenesis, which are contributed to our better understanding of cancer pathogenesis and therapeutics. Indole alkaloids derived from natural sources are well defined for their outstanding biological and pharmacological properties, like vincristine, vinblastine, staurosporine, indirubin, and 3,3'-diindolylmethane, which are currently used in the clinic or under clinical assessment. Moreover, such compounds play a significant role in discovering novel anticancer agents. Thus, here we systemically summarized recent advances in indole alkaloids as anticancer agents by targeting different RCD subroutines, including the classical apoptosis and autophagic cell death signaling pathways as well as the crucial signaling pathways of other RCD subroutines, such as ferroptosis, mitotic catastrophe, necroptosis, and anoikis, in cancer. Moreover, we further discussed the cross talk between different RCD subroutines mediated by indole alkaloids and the combined strategies of multiple agents (e.g., 3,10-dibromofascaplysin combined with olaparib) to exhibit therapeutic potential against various cancers by regulating RCD subroutines. In short, the information provided in this review on the regulation of cell death by indole alkaloids against different targets is expected to be beneficial for the design of novel molecules with greater targeting and biological properties, thereby facilitating the development of new strategies for cancer therapy.
Keywords: Anoikis; Apoptosis; Autophagy; Cancer; Ferroptosis; Indole alkaloids; Mitotic catastrophe; Necroptosis; Regulated cell death (RCD); Target therapy.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Targeting regulated cell death (RCD) with small-molecule compounds in triple-negative breast cancer: a revisited perspective from molecular mechanisms to targeted therapies.J Hematol Oncol. 2022 Apr 12;15(1):44. doi: 10.1186/s13045-022-01260-0. J Hematol Oncol. 2022. PMID: 35414025 Free PMC article. Review.
-
Regulated cell death (RCD) in cancer: key pathways and targeted therapies.Signal Transduct Target Ther. 2022 Aug 13;7(1):286. doi: 10.1038/s41392-022-01110-y. Signal Transduct Target Ther. 2022. PMID: 35963853 Free PMC article. Review.
-
Targeting regulated cell death with plant natural compounds for cancer therapy: A revisited review of apoptosis, autophagy-dependent cell death, and necroptosis.Phytother Res. 2023 Apr;37(4):1488-1525. doi: 10.1002/ptr.7738. Epub 2023 Jan 30. Phytother Res. 2023. PMID: 36717200 Review.
-
Targeting regulated cell death (RCD) with small-molecule compounds in cancer therapy: A revisited review of apoptosis, autophagy-dependent cell death and necroptosis.Drug Discov Today. 2022 Feb;27(2):612-625. doi: 10.1016/j.drudis.2021.10.011. Epub 2021 Oct 27. Drug Discov Today. 2022. PMID: 34718209 Review.
-
Targeting regulated cell death (RCD) in hematological malignancies: Recent advances and therapeutic potential.Biomed Pharmacother. 2024 Jun;175:116667. doi: 10.1016/j.biopha.2024.116667. Epub 2024 May 3. Biomed Pharmacother. 2024. PMID: 38703504 Review.
Cited by
-
Dysregulated CREB3 cleavage at the nuclear membrane induces karyoptosis-mediated cell death.Exp Mol Med. 2024 Mar;56(3):686-699. doi: 10.1038/s12276-024-01195-1. Epub 2024 Mar 13. Exp Mol Med. 2024. PMID: 38480902 Free PMC article.
-
Evaluation of Vincamine Loaded with Silver Nanoparticles as a New Potential Therapeutic Agent Against Ehrlich's Solid Carcinoma in Mice.Cells. 2024 Oct 24;13(21):1762. doi: 10.3390/cells13211762. Cells. 2024. PMID: 39513869 Free PMC article.
-
The Protective Effect of (-)-Tetrahydroalstonine against OGD/R-Induced Neuronal Injury via Autophagy Regulation.Molecules. 2023 Mar 4;28(5):2370. doi: 10.3390/molecules28052370. Molecules. 2023. PMID: 36903613 Free PMC article.
-
Molecular characteristics and prognostic significances of lysosomal-dependent cell death in kidney renal clear cell carcinoma.Aging (Albany NY). 2024 Mar 7;16(5):4862-4888. doi: 10.18632/aging.205639. Epub 2024 Mar 7. Aging (Albany NY). 2024. PMID: 38460947 Free PMC article.
-
Exploiting Nanotechnology for Drug Delivery: Advancing the Anti-Cancer Effects of Autophagy-Modulating Compounds in Traditional Chinese Medicine.Int J Nanomedicine. 2024 Mar 12;19:2507-2528. doi: 10.2147/IJN.S455407. eCollection 2024. Int J Nanomedicine. 2024. PMID: 38495752 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

