Novel tricyclic small molecule inhibitors of Nicotinamide N-methyltransferase for the treatment of metabolic disorders
- PMID: 36104373
- PMCID: PMC9474883
- DOI: 10.1038/s41598-022-19634-2
Novel tricyclic small molecule inhibitors of Nicotinamide N-methyltransferase for the treatment of metabolic disorders
Abstract
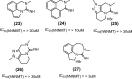
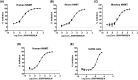
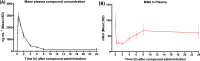
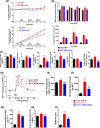
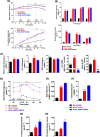
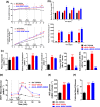
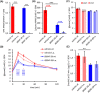
Nicotinamide N-methyltransferase (NNMT) is a metabolic regulator that catalyzes the methylation of nicotinamide (Nam) using the co-factor S-adenosyl-L-methionine to form 1-methyl-nicotinamide (MNA). Overexpression of NNMT and the presence of the active metabolite MNA is associated with a number of diseases including metabolic disorders. We conducted a high-throughput screening campaign that led to the identification of a tricyclic core as a potential NNMT small molecule inhibitor series. Elaborate medicinal chemistry efforts were undertaken and hundreds of analogs were synthesized to understand the structure activity relationship and structure property relationship of this tricyclic series. A lead molecule, JBSNF-000028, was identified that inhibits human and mouse NNMT activity, reduces MNA levels in mouse plasma, liver and adipose tissue, and drives insulin sensitization, glucose modulation and body weight reduction in a diet-induced obese mouse model of diabetes. The co-crystal structure showed that JBSNF-000028 binds below a hairpin structural motif at the nicotinamide pocket and stacks between Tyr-204 (from Hairpin) and Leu-164 (from central domain). JBSNF-000028 was inactive against a broad panel of targets related to metabolism and safety. Interestingly, the improvement in glucose tolerance upon treatment with JBSNF-000028 was also observed in NNMT knockout mice with diet-induced obesity, pointing towards the glucose-normalizing effect that may go beyond NNMT inhibition. JBSNF-000028 can be a potential therapeutic option for metabolic disorders and developmental studies are warranted.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
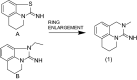
Similar articles
-
A small molecule inhibitor of Nicotinamide N-methyltransferase for the treatment of metabolic disorders.Sci Rep. 2018 Feb 26;8(1):3660. doi: 10.1038/s41598-018-22081-7. Sci Rep. 2018. PMID: 29483571 Free PMC article.
-
Selective and membrane-permeable small molecule inhibitors of nicotinamide N-methyltransferase reverse high fat diet-induced obesity in mice.Biochem Pharmacol. 2018 Jan;147:141-152. doi: 10.1016/j.bcp.2017.11.007. Epub 2017 Nov 15. Biochem Pharmacol. 2018. PMID: 29155147 Free PMC article.
-
Nicotinamide N-methyltransferase: At the crossroads between cellular metabolism and epigenetic regulation.Mol Metab. 2021 Mar;45:101165. doi: 10.1016/j.molmet.2021.101165. Epub 2021 Jan 14. Mol Metab. 2021. PMID: 33453420 Free PMC article. Review.
-
Novel Inhibitors of Nicotinamide-N-Methyltransferase for the Treatment of Metabolic Disorders.Molecules. 2021 Feb 13;26(4):991. doi: 10.3390/molecules26040991. Molecules. 2021. PMID: 33668468 Free PMC article.
-
Nicotinamide N-methyl transferase (NNMT): An emerging therapeutic target.Drug Discov Today. 2021 Nov;26(11):2699-2706. doi: 10.1016/j.drudis.2021.05.011. Epub 2021 May 21. Drug Discov Today. 2021. PMID: 34029690 Review.
Cited by
-
Diabetes Mellitus and Pregnancy: An Insight into the Effects on the Epigenome.Biomedicines. 2024 Feb 2;12(2):351. doi: 10.3390/biomedicines12020351. Biomedicines. 2024. PMID: 38397953 Free PMC article. Review.
-
Epigenetic Modulators as Therapeutic Agents in Cancer.Int J Mol Sci. 2023 Oct 6;24(19):14964. doi: 10.3390/ijms241914964. Int J Mol Sci. 2023. PMID: 37834411 Free PMC article. Review.
-
Epigenetic Regulation in Oral Squamous Cell Carcinoma Microenvironment: A Comprehensive Review.Cancers (Basel). 2023 Nov 27;15(23):5600. doi: 10.3390/cancers15235600. Cancers (Basel). 2023. PMID: 38067304 Free PMC article. Review.
-
NAD+ Homeostasis and NAD+-Consuming Enzymes: Implications for Vascular Health.Antioxidants (Basel). 2023 Feb 4;12(2):376. doi: 10.3390/antiox12020376. Antioxidants (Basel). 2023. PMID: 36829935 Free PMC article. Review.
-
The Role of NAD+ and NAD+-Boosting Therapies in Inflammatory Response by IL-13.Pharmaceuticals (Basel). 2024 Feb 8;17(2):226. doi: 10.3390/ph17020226. Pharmaceuticals (Basel). 2024. PMID: 38399441 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

