RACK1 Regulates Poxvirus Protein Synthesis Independently of Its Role in Ribosome-Based Stress Signaling
- PMID: 36098514
- PMCID: PMC9517738
- DOI: 10.1128/jvi.01093-22
RACK1 Regulates Poxvirus Protein Synthesis Independently of Its Role in Ribosome-Based Stress Signaling
Abstract
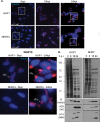
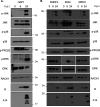
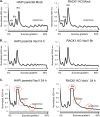
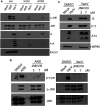
Receptor for activated C kinase 1 (RACK1) is a small ribosomal subunit protein that is phosphorylated by vaccinia virus (VacV) to maximize translation of postreplicative (PR) mRNAs that harbor 5' polyA leaders. However, RACK1 is a multifunctional protein that both controls translation directly and acts as a scaffold for signaling to and from the ribosome. This includes stress signaling that is activated by ribosome-associated quality control (RQC) and ribotoxic stress response (RSR) pathways. As VacV infection activates RQC and stress signaling, whether RACK1 influences viral protein synthesis through its effects on translation, signaling, or both remains unclear. Examining the effects of genetic knockout of RACK1 on the phosphorylation of key mitogenic and stress-related kinases, we reveal that loss of RACK1 specifically blunts the activation of c-Jun N-terminal kinase/stress-activated protein kinase (JNK/SAPK) at late stages of infection. However, RACK1 was not required for JNK recruitment to ribosomes, and unlike RACK1 knockout, JNK inhibitors had no effect on viral protein synthesis. Moreover, reduced JNK activity during infection in RACK1 knockout cells contrasted with the absolute requirement for RACK1 in RSR-induced JNK phosphorylation. Comparing the effects of RACK1 knockout alongside inhibitors of late stage replication, our data suggest that JNK activation is only indirectly affected by the absence of RACK1 due to reduced viral protein accumulation. Cumulatively, our findings in the context of infection add further support for a model whereby RACK1 plays a specific and direct role in controlling translation of PR viral mRNAs that is independent of its role in ribosome-based stress signaling. IMPORTANCE Receptor for activated C kinase 1 (RACK1) is a multifunctional ribosomal protein that regulates translation directly and mediates signaling to and from the ribosome. While recent work has shown that RACK1 is phosphorylated by vaccinia virus (VacV) to stimulate translation of postreplicative viral mRNAs, whether RACK1 also contributes to VacV replication through its roles in ribosome-based stress signaling remains unclear. Here, we characterize the role of RACK1 in infected cells. In doing so, we find that RACK1 is essential for stress signal activation by ribotoxic stress responses but not by VacV infection. Moreover, although the loss of RACK1 reduces the level of stress-associated JNK activation in infected cells, this is an indirect consequence of RACK1's specific requirement for the synthesis of postreplicative viral proteins, the accumulation of which determines the level of cellular stress. Our findings reveal both the specific role of RACK1 and the complex downstream effects of its control of viral protein synthesis in the context of infection.
Keywords: RACK1; poxvirus; ribosome; ribosome quality control; ribotoxic stress responses; signaling; stress; stress signaling; translation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Trans-kingdom mimicry underlies ribosome customization by a poxvirus kinase.Nature. 2017 Jun 29;546(7660):651-655. doi: 10.1038/nature22814. Epub 2017 Jun 21. Nature. 2017. PMID: 28636603 Free PMC article.
-
Ribosome customization and functional diversification among P-stalk proteins regulate late poxvirus protein synthesis.Cell Rep. 2025 Jan 28;44(1):115119. doi: 10.1016/j.celrep.2024.115119. Epub 2025 Jan 8. Cell Rep. 2025. PMID: 39786991 Free PMC article.
-
Communication between RACK1/Asc1 and uS3 (Rps3) is essential for RACK1/Asc1 function in yeast Saccharomyces cerevisiae.Gene. 2019 Jul 20;706:69-76. doi: 10.1016/j.gene.2019.04.087. Epub 2019 May 1. Gene. 2019. PMID: 31054365 Free PMC article.
-
Structural analysis of ribosomal RACK1 and its role in translational control.Cell Signal. 2017 Jul;35:272-281. doi: 10.1016/j.cellsig.2017.01.026. Epub 2017 Feb 2. Cell Signal. 2017. PMID: 28161490 Review.
-
Regulation of eukaryotic translation by the RACK1 protein: a platform for signalling molecules on the ribosome.EMBO Rep. 2004 Dec;5(12):1137-41. doi: 10.1038/sj.embor.7400291. EMBO Rep. 2004. PMID: 15577927 Free PMC article. Review.
Cited by
-
Ribosomes in poxvirus infection.Curr Opin Virol. 2022 Oct;56:101256. doi: 10.1016/j.coviro.2022.101256. Epub 2022 Oct 18. Curr Opin Virol. 2022. PMID: 36270183 Free PMC article. Review.
-
The poxvirus F17 protein counteracts mitochondrially orchestrated antiviral responses.Nat Commun. 2023 Nov 30;14(1):7889. doi: 10.1038/s41467-023-43635-y. Nat Commun. 2023. PMID: 38036506 Free PMC article.
-
Multi-omics characterization of the monkeypox virus infection.Nat Commun. 2024 Aug 8;15(1):6778. doi: 10.1038/s41467-024-51074-6. Nat Commun. 2024. PMID: 39117661 Free PMC article.
-
YTHDF2 Is Downregulated in Response to Host Shutoff Induced by DNA Virus Infection and Regulates Interferon-Stimulated Gene Expression.J Virol. 2023 Mar 30;97(3):e0175822. doi: 10.1128/jvi.01758-22. Epub 2023 Mar 14. J Virol. 2023. PMID: 36916936 Free PMC article.
-
Repurposing Anti-Dengue Compounds against Monkeypox Virus Targeting Core Cysteine Protease.Biomedicines. 2023 Jul 18;11(7):2025. doi: 10.3390/biomedicines11072025. Biomedicines. 2023. PMID: 37509664 Free PMC article.
References
-
- Moss B. 2007. Poxviridae: the viruses and their replication, p 2849–2883. In Knipe DM, Howley PM (ed), Fields virology. Lippincott Williams & Wilkins, Philadelphia, PA.
-
- Simpson K, Heymann D, Brown CS, Edmunds WJ, Elsgaard J, Fine P, Hochrein H, Hoff NA, Green A, Ihekweazu C, Jones TC, Lule S, Maclennan J, McCollum A, Muhlemann B, Nightingale E, Ogoina D, Ogunleye A, Petersen B, Powell J, Quantick O, Rimoin AW, Ulaeato D, Wapling A. 2020. Human monkeypox—after 40 years, an unintended consequence of smallpox eradication. Vaccine 38:5077–5081. 10.1016/j.vaccine.2020.04.062. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

