Salivary orosomucoid 1 as a biomarker of hepatitis B associated hepatocellular carcinoma
- PMID: 36096917
- PMCID: PMC9467997
- DOI: 10.1038/s41598-022-18894-2
Salivary orosomucoid 1 as a biomarker of hepatitis B associated hepatocellular carcinoma
Abstract
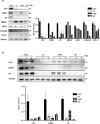
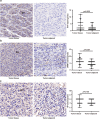
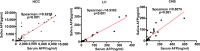
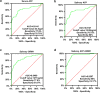
Saliva is rich in proteins, DNA, RNA and microorganisms, and can be regarded as a biomarker library. In order to explore a noninvasive and simple means of early screening for liver cancer, proteomics was used to screen salivary markers of hepatitis B associated liver cancer. We used mass spectrometry coupled isobaric tags for relative and absolute quantitation (iTRAQ)-technology to identify differentially expressed proteins (DEPs). Western blot, immunohistochemistry and enzyme linked immunosorbent assay were used to detect marker expression of in tissues and saliva. Statistical analysis was used to analyze the diagnostic efficacy of the markers was analyzed through statistical analyses. By comparing the hepatocellular carcinoma (HCC) group with non-HCC groups, we screened out 152 salivary DEPs. We found orosomucoid 1(ORM1) had significantly higher expression in saliva of HCC patients compared with non-HCC groups (p < 0.001) and the expression of ORM1 in liver cancer tissues was significantly higher than that in adjacent normal tissues (p < 0.001). The combination of salivary ORM1 and alpha-fetoprotein (AFP) showed reasonable specificities and sensitivities for detecting HCC. In a word, salivary ORM1 as a new biomarker of hepatitis B associated hepatocellular carcinoma, combination of salivary ORM1 and AFP as an improved diagnostic tool for hepatocellular carcinoma.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
[Research and exploration of salivary biological markers for hepatitis B-related hepatocellular carcinoma].Zhonghua Gan Zang Bing Za Zhi. 2023 May 20;31(5):495-503. doi: 10.3760/cma.j.cn501113-20230210-00047. Zhonghua Gan Zang Bing Za Zhi. 2023. PMID: 37365026 Chinese.
-
Urine α-fetoprotein and orosomucoid 1 as biomarkers of hepatitis B virus-associated hepatocellular carcinoma.Am J Physiol Gastrointest Liver Physiol. 2020 Feb 1;318(2):G305-G312. doi: 10.1152/ajpgi.00267.2019. Epub 2019 Nov 18. Am J Physiol Gastrointest Liver Physiol. 2020. PMID: 31736338
-
Screening for Lipid-Metabolism-Related Genes and Identifying the Diagnostic Potential of ANGPTL6 for HBV-Related Early-Stage Hepatocellular Carcinoma.Biomolecules. 2022 Nov 17;12(11):1700. doi: 10.3390/biom12111700. Biomolecules. 2022. PMID: 36421714 Free PMC article.
-
Proteomics in the diagnosis of hepatocellular carcinoma: focus on high risk hepatitis B and C patients.Anticancer Res. 2006 Sep-Oct;26(5A):3293-300. Anticancer Res. 2006. PMID: 17094443 Review.
-
Circulating predictive and diagnostic biomarkers for hepatitis B virus-associated hepatocellular carcinoma.World J Gastroenterol. 2016 Oct 7;22(37):8271-8282. doi: 10.3748/wjg.v22.i37.8271. World J Gastroenterol. 2016. PMID: 27729734 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

