Putative COVID-19 therapies imatinib, lopinavir, ritonavir, and ivermectin cause hair cell damage: A targeted screen in the zebrafish lateral line
- PMID: 36090793
- PMCID: PMC9448854
- DOI: 10.3389/fncel.2022.941031
Putative COVID-19 therapies imatinib, lopinavir, ritonavir, and ivermectin cause hair cell damage: A targeted screen in the zebrafish lateral line
Abstract
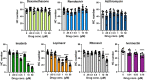
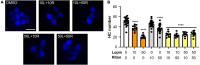
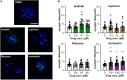
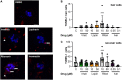
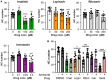
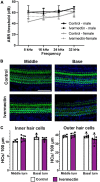
The biomedical community is rapidly developing COVID-19 drugs to bring much-need therapies to market, with over 900 drugs and drug combinations currently in clinical trials. While this pace of drug development is necessary, the risk of producing therapies with significant side-effects is also increased. One likely side-effect of some COVID-19 drugs is hearing loss, yet hearing is not assessed during preclinical development or clinical trials. We used the zebrafish lateral line, an established model for drug-induced sensory hair cell damage, to assess the ototoxic potential of seven drugs in clinical trials for treatment of COVID-19. We found that ivermectin, lopinavir, imatinib, and ritonavir were significantly toxic to lateral line hair cells. By contrast, the approved COVID-19 therapies dexamethasone and remdesivir did not cause damage. We also did not observe damage from the antibiotic azithromycin. Neither lopinavir nor ritonavir altered the number of pre-synaptic ribbons per surviving hair cell, while there was an increase in ribbons following imatinib or ivermectin exposure. Damage from lopinavir, imatinib, and ivermectin was specific to hair cells, with no overall cytotoxicity noted following TUNEL labeling. Ritonavir may be generally cytotoxic, as determined by an increase in the number of TUNEL-positive non-hair cells following ritonavir exposure. Pharmacological inhibition of the mechanotransduction (MET) channel attenuated damage caused by lopinavir and ritonavir but did not alter imatinib or ivermectin toxicity. These results suggest that lopinavir and ritonavir may enter hair cells through the MET channel, similar to known ototoxins such as aminoglycoside antibiotics. Finally, we asked if ivermectin was ototoxic to rats in vivo. While ivermectin is not recommended by the FDA for treating COVID-19, many people have chosen to take ivermectin without a doctor's guidance, often with serious side-effects. Rats received daily subcutaneous injections for 10 days with a clinically relevant ivermectin dose (0.2 mg/kg). In contrast to our zebrafish assays, ivermectin did not cause ototoxicity in rats. Our research suggests that some drugs in clinical trials for COVID-19 may be ototoxic. This work can help identify drugs with the fewest side-effects and determine which therapies warrant audiometric monitoring.
Keywords: COVID-19 therapy; hair cell; ivermectin; lateral line; ototoxicity; remdesivir; zebrafish.
Copyright © 2022 Coffin, Dale, Doppenberg, Fearington, Hayward, Hill and Molano.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Detecting Novel Ototoxins and Potentiation of Ototoxicity by Disease Settings.Front Neurol. 2021 Aug 17;12:725566. doi: 10.3389/fneur.2021.725566. eCollection 2021. Front Neurol. 2021. PMID: 34489859 Free PMC article. Review.
-
Molecular Docking of Azithromycin, Ritonavir, Lopinavir, Oseltamivir, Ivermectin and Heparin Interacting with Coronavirus Disease 2019 Main and Severe Acute Respiratory Syndrome Coronavirus-2 3C-Like Proteases.J Nanosci Nanotechnol. 2021 Apr 1;21(4):2075-2089. doi: 10.1166/jnn.2021.19029. J Nanosci Nanotechnol. 2021. PMID: 33500022
-
Using the zebrafish lateral line to screen for ototoxicity.J Assoc Res Otolaryngol. 2008 Jun;9(2):178-90. doi: 10.1007/s10162-008-0118-y. Epub 2008 Apr 12. J Assoc Res Otolaryngol. 2008. PMID: 18408970 Free PMC article.
-
Hair cell toxicity in anti-cancer drugs: evaluating an anti-cancer drug library for independent and synergistic toxic effects on hair cells using the zebrafish lateral line.J Assoc Res Otolaryngol. 2011 Dec;12(6):719-28. doi: 10.1007/s10162-011-0278-z. Epub 2011 Jul 6. J Assoc Res Otolaryngol. 2011. PMID: 21732171 Free PMC article.
-
Lopinavir/ritonavir: a review of its use in the management of HIV infection.Drugs. 2003;63(8):769-802. doi: 10.2165/00003495-200363080-00004. Drugs. 2003. PMID: 12662125 Review.
Cited by
-
Post Covid telogen effluvium: the diagnostic value of serum ferritin biomarker and the preventive value of dietary supplements. a case control study.Arch Dermatol Res. 2024 Jun 6;316(6):336. doi: 10.1007/s00403-024-03004-1. Arch Dermatol Res. 2024. PMID: 38844670 Free PMC article.
-
Larval zebrafish maintain elevation with multisensory control of posture and locomotion.bioRxiv [Preprint]. 2024 Dec 20:2024.01.23.576760. doi: 10.1101/2024.01.23.576760. bioRxiv. 2024. PMID: 38328242 Free PMC article. Preprint.
-
Identification of side effects of COVID-19 drug candidates on embryogenesis using an integrated zebrafish screening platform.Sci Rep. 2023 Oct 9;13(1):17037. doi: 10.1038/s41598-023-43911-3. Sci Rep. 2023. PMID: 37813860 Free PMC article.
-
Hair cell toxicology: With the help of a little fish.Front Cell Dev Biol. 2022 Dec 13;10:1085225. doi: 10.3389/fcell.2022.1085225. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36582469 Free PMC article. Review.
-
A screen of pharmacologically active compounds to identify modulators of the Adgrg6/Gpr126 signalling pathway in zebrafish embryos.Basic Clin Pharmacol Toxicol. 2023 Oct;133(4):364-377. doi: 10.1111/bcpt.13923. Epub 2023 Jul 16. Basic Clin Pharmacol Toxicol. 2023. PMID: 37394692 Free PMC article.
References
-
- Ader F., Peiffer-Smadja N., Poissy J., Bouscambert-Duchamp M., Belhadi D., Diallo A., et al. (2021). An open-label randomized controlled trial of the effect of lopinavir/ritonavir, lopinavir/ritonavir plus IFN-β-1a and hydroxychloroquine in hospitalized patients with COVID-19. Clin. Microbiol. Infect. 27 1826–1837. 10.1016/j.cmi.2021.05.020 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

